doi: 10.62486/agmu2024102
ORIGINAL
Active packaging technology: cassava starch/orange essential oil for antimicrobial food packaging
Tecnología de empaques activos: almidón de yuca/ aceite esencial de naranja para el envasado antimicrobiano de alimentos
Olga Lucia Torres Vargas1 *, Iván Andrés Rodríguez Agredo1
1Universidad del Quindío, Grupo de Investigación en Ciencias Agroindustriales (GICA) Instituto Interdisciplinario de las Ciencias, Laboratorio de Ingeniería de Alimentos, Armenia, Quindío. Colombia.
Cite as: Torres Vargas OL, Rodríguez Agredo IA. Active packaging technology: cassava starch/orange essential oil for antimicrobial food packaging. Multidisciplinar (Montevideo). 2024; 2:102. https://doi.org/10.62486/agmu2024102
Submitted: 24-12-2023 Revised: 03-04-2024 Accepted: 31-07-2024 Published: 01-08-2024
ABSTRACT
New technologies for active food packaging that can protect and interact with the food, increasing its shelf life are currently being developed. Essential oils are active compounds that, in addition to providing antibacterial protection, can improve the functional and mechanical properties of films. This research aimed to evaluate the influence of orange (Citrus sinensis L.) essential oil (AEN) on the physical and antimicrobial properties of active films produced from cassava (Manihot esculenta) starch and alginate (AY/AG) using the plate diffusion technique. The films were formulated with different concentrations of AEN (0, 0,5, 1,0 and 1,5 %). Elongation at break (EB), water vapor permeability (WVP), moisture content, solubility and Luminosity (L*) decreased significantly (p < 0,05) with the addition of AEN, on the other hand, tensile strength (TS), b* value (tendency towards yellow) and opacity increased. Scanning electron microscopy (SEM) images showed a smooth, uniform appearance and continuous dispersion between cassava starch, alginate. The results obtained indicated that the incorporation of AEN presented an inhibitory effect against Escherichia coli and Staphylococcus aureus bacteria. Therefore, the films obtained have a high potential to be used in the development of antimicrobial packaging for food applications.
Keywords: Orange Essential Oil; Antimicrobial Activity; Starch Films; Physical Properties.
RESUMEN
Nuevas tecnologías para el envasado activo de alimentos que pueden proteger e interactuar con el alimento, aumentando su vida útil se están desarrollando en la actualidad. Los aceites esenciales son compuestos activos que, además de brindar protección antibacteriana, pueden mejorar las propiedades funcionales y mecánicas de las películas. Esta investigación tuvo como objetivo evaluar la influencia del aceite de esencial de naranja (Citrus sinensis L.) (AEN) en las propiedades físicas y antimicrobianas de películas activas producidas a partir de almidón de yuca (Manihot esculenta) y alginato (AY/AG) mediante la técnica de difusión en placa. Las películas fueron formuladas con diferentes concentraciones de AEN (0, 0,5, 1,0 y 1,5 %). El alargamiento a la rotura (EB), la permeabilidad al vapor de agua (WVP), el contenido de humedad, la solubilidad y la Luminosidad (L*) disminuyeron significativamente (p < 0,05) con la adición de AEN, en cambio, la resistencia a la tracción (TS), el valor de b* (tendencia hacia el amarillo) y la opacidad aumentaron. Las imágenes de microscopia electrónica de barrido (SEM) mostraron una apariencia lisa, uniforme y una dispersión continua entre el almidón de yuca, el alginato. Los resultados obtenidos indicaron que la incorporación de AEN presento un efecto inhibidor contra las bacterias Escherichia coli y Staphylococcus aureus. Por lo tanto, las películas obtenidas, tienen un alto potencial para ser utilizadas en el desarrollo de empaques antimicrobianas para aplicaciones alimentarias.
Palabras clave: Aceite Esencial de Naranja; Actividad Antimicrobiana; Películas de Almidón; Propiedades Físicas.
INTRODUCTION
Antimicrobial compounds are incorporated into edible films as bioactive components to improve food safety and quality. Essential oils exhibit strong antimicrobial properties and can improve the film’s functional and mechanical properties (Sadaf & Idrees, 2022).
Prolonged quality, safety, and shelf life of agricultural products increase consumer acceptability. Edible films represent an alternative solution for the preservation of minimally processed vegetables. Coating materials with natural antimicrobials can be an opportunity to increase the safety of fresh produce. Edible films also control the disadvantages of essential oils in vegetable preservation (Yousuf et al., 2021; Zhu et al., 2021).
Films formulated with active ingredients of natural origin are promising for biodegradable packaging and their use in food preservation; they represent an environmentally friendly alternative to the packaging traditionally used in the food industry (Jafarzadeha et al., 2020). Starch, a natural, low-cost, biodegradable polysaccharide with good biocompatibility, can be used as a carrier of bioactive substances that improve the function of food packaging (Menzel, C. 2020). As a plasticizer, glycerol can reduce the cohesion between molecules and increase the fluidity of the polymer chain, thus improving the flexibility of the film and reducing its brittleness (Chillo et al., 2008). Essential oils have an oily and volatile nature that can affect the integrity or degree of hydrophobicity of polymer films, changing their mechanical and barrier properties (Atarés & Chiralt, 2016). Therefore, studies are needed to examine the potential of each antibacterial agent and its interaction with the material used to produce the active starch films.
Orange essential oil (AEN) obtained from the citrus peel (Citrus sinensis L.) has strong antimicrobial properties, which could be due to the high quantity relative amount of terpenic compounds, including D-limonene (90 % -96 %), myrcene (19 %), and linalool (0,32 %) (Silveira et al., 2021). It has attracted special attention in scientific communities for its potential applications as a natural preservative (Aguiar et al., 2020). The FDA recognized it as safe (GRAS) in 2018. it is used as a preservative or flavoring agent in food industries (Sharma & Tripathi, 2008) and has become an ideal choice for incorporation in starch-formulated active films (Tan et al., 2019); it not only improves the antimicrobial and antioxidant capabilities of films but also functions as a bulking agent in the film forming solution (SFP), improving mechanical and physical properties (Sahraee et al., 2019). However, more information is needed on its activity in the cassava starch/Alginate mixture or its impact on the functional properties of the film. Therefore, this study aimed to formulate cassava starch/alginate-based films active with AEN and to study their effect on the physical and antimicrobial properties of the obtained films.
METHOD
Materials
Cassava starch was used as the film matrix forming component to prepare the films, which was acquired from TECNAS, Medellín, Colombia. Sodium alginate and glycerol were purchased from Sigma-Aldrich, USA, and AEN from Natuaroma S.A., Colombia. The bacterial strains S.aureus Gram (+) and E coli Gram (-) were acquired from Merck, Colombia.
Gas Chromatography-Mass Spectrometry (GC-MS) analysis
The chemical composition of the AEN was determined by gas chromatography-mass spectrometry (GC-MS). An Agilent HP- 6890N gas chromatograph coupled to an Agilent 5975N mass selective detector (Agilent et al. USA) was used, and compound identification was based on mass spectra (MS) obtained with NIST02.L, NIST5a.L, and NIST98.L library data and the selected ion monitoring mode was used to determine compound concentrations.
Minimum inhibitory concentration (MIC)
To determine the minimum inhibitory concentration (MIC) of AEM, a Gram-negative Escherichia coli (ATCC 25922) and a Gram-positive Staphylococcus aureus (ATCC 25923) bacteria obtained from the GYMOL Group of the University of Quindío were selected. The strains were previously cultured in Luria Bertani (LB) medium (10g/L tryptone, 5g/L yeast extract, and 5g/L NaCl) for 24h at 36± one °C. After this period, the bacterial suspension corresponded to approximately 108 CFU/mL. MIC assays were performed with concentrations between 0,01 and 5mg/mL of AEN in microplates with a volume of 200 μL of LB medium and ten μL of bacteria in each dilution. Absorbance readings were taken at 490 nm before and after incubation for 24h at 36 ± 1 °C. MIC was defined as the lowest concentration of AEN in mg/mL capable of inhibiting microbial growth, measured by the difference between each bacterium’s final and initial absorbance.
Preparation of films
Films formulated with cassava starch were obtained by the plate diffusion or casting method following the methodology proposed by Torres et al., 2021 with some modifications. The film-forming solution (FFS) was prepared by homogenizing the following components: A 1,8 % (w/w) aqueous suspension of cassava starch at 60°C for 30 min at 600 rpm, 1,2 % (w/w) sodium alginate at 600 rpm with constant stirring. The amounts of glycerol (15 %) and calcium chloride (1,7 %) were calculated based on the mass of sodium alginate. Subsequently, the calcium chloride solution was added to the sodium alginate solution, and a glycerol solution was heated for 15 minutes. All components were mixed to obtain the SFP. Subsequently, AEN (0,0, 0,5, 1,0, and 1,5 %) was added to the SFP. Once homogenized, the SFP was poured into the molds and dried in a forced air oven (Binder et al.) until the films were obtained. All films were stored at 20°C in a desiccator with a relative humidity of 50 % until further characterization. A control film (PC) will be formulated using the same methodology but without incorporating AEN.
Film characterization
Thickness
The thickness of the formulated films was measured following the methodology described by (Siripatrawan and Harte, 2010) with some modifications. A digital micrometer with an accuracy of 0,001 mm (Mitutoyo et al.) was used for the measurements. Ten measurements were taken at randomly selected points on each type of film obtained; the value of each thickness will be the average of the measurements taken.
Moisture content
Moisture content was determined immediately after the formulated films were obtained by conditioning for 48h at 25°C at a relative humidity of 50 %, following the ASTM D644-99 method. Moisture was determined by the gravimetric method, and the samples were cut into 2x2 cm sheets and weighed. Initially, the weight of the films was taken, and then they were placed in an oven with forced air at 105°C for 24 hours until they were constantly weighed.
Solubility
The solubility of the formulated films was determined by the percentage of the film dry matter that is soluble in water. The methodology reported by Akhter et al., 2019 was followed with some modifications. The formulated films were cut into 2 x 2cm sheets. The samples were dried at 105°C to constant weight to obtain the initial dry mass. The formulated films were placed in 50 mL of distilled water, covered, and stored at 25°C for 24h, then vacuum filtered and dried at 105°C to constant weight to obtain the final dry mass.
Water vapor permeability (PVA)
To determine the water vapor permeability (WVP), the formulated films were conditioned for 48 hours at 20 ± 0,1 ºC and a relative humidity of 40 ± 1 %. Once conditioned, they were evaluated according to ASTM E-96 (2005).
The capsules were weighed every hour for 9 hours to determine the film’s weight loss.
Film color and opacity
The color and opacity of the films were determined using a colorimeter (MINOLTA, CR 400, Japan). The films were placed on a white plate, defined as standard, and illuminance D65 (daylight) was used to determine the color parameters. The L* parameter indicates lightness, ranging from 0 (black) to 100 (white); the a* and b* parameters are the chromaticity coordinates, where a* ranges from green (-) to red (+) and b* ranges from (-) to yellow (+).
Scanning Electron Microscopy (SEM)
The morphological characterization of the surface and cross-section of the obtained films was performed by scanning electron microscopy (SEM). The films were cut into 1-2 mm long sheets. The formulated films were fixed with conductive carbon and metalized with gold to present electrically conductive properties. A JSM-6610LV microscope, JEOL Ltd Japan, was used, and the formulated films were systematically observed at magnifications of 10000x using an accelerating voltage of 5 KV and 500x using an accelerating voltage of 10 KV.
Antimicrobial activity
The antimicrobial activity of the films was evaluated using the disk diffusion method with modifications described by (Bajpai et al., 2011) with slight modifications. Disks of 5 mm diameter were aseptically cut and placed in Petri dishes with solid Mueller-Hinton agar and swabbed with 1,0mL of 10-7 (100μl) CFU/mL of Escherichia coli and Staphylococcus aureus bacterial culture suspensions. The plates were incubated at 37,0 ± 0,1 °C for 24 hours. The diameter of the inhibition zones of the discs was measured using a digital micrometer. Each sample was evaluated in triplicate, and the average and standard deviation of the measurements were used as the result.
Statistical analysis
All determinations were made in triplicate, and the results were reported as the mean ± standard deviation. Statistical data analysis was performed using variance analysis (ANOVA) and Statgraphics Centurion XVIII software. Differences between the mean values of film properties were compared using Tukey’s test with a 95 % confidence level (p<0,05). The Shapiro-Wilks test was used to verify the normality and homogeneity fit of the variance data.
RESULTS AND DISCUSSION
Chemical Analysis: Orange Essential Oil
GC-MS analysis identified the presence of seven components in AEN. The main compounds were 84 % d-limonene and 2,1 % β-myrcene, the main constituents of AEL. Five other compounds were also identified in lower concentrations: 0,50 % octanal, 0,42 % α-pinene, 0,45 % β-linalool, 0,20 % cyclohexene, and 0,20 % decanal; these results agree with those reported by Do Evangelho, (2019).
Minimum inhibitory concentration (MIC).
The results obtained for the antimicrobial activity of AEN against two bacterial strains. Revealed that AEM presented the lowest minimum inhibitory concentration (MIC) of 0,025 mg/mL for the large negative Escherichia coli strain and 0,050 mg/mL for the gram-positive Staphylococcus aureus strain. Therefore, the Gram-positive bacteria were more sensitive to AEM than the Gram-negative strain. The findings are consistent with previous studies published by Singh et al. (2015), where it was observed that MIC showed higher efficacy against gram-positive bacteria than gram-negative bacteria.
Morphological analysis of films
In figure 1, the images of the visual appearance and SEM photographs made to the PC and PA/AY/AEN-1,5 % film are presented. As shown in figure 1 a), the films presented a cohesive matrix that was continuous and flexible to handle, with smooth surfaces without cracks that could lead to breakage or insoluble particles that could be observed with the naked eye.
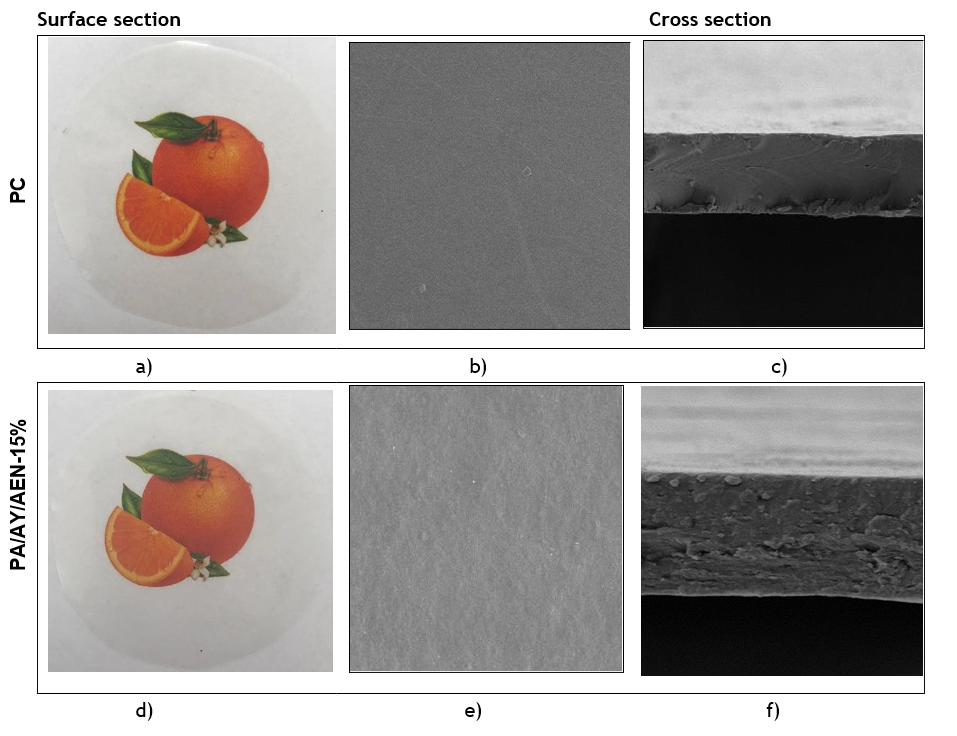
Figure 1. Images of the visual appearance and SEM photographs of PC and PA/AY/AEN-1.5% film
In figure 1 b) the incorporation of AEN in PA/AY/AEN, regardless of the concentration, reduced the homogeneity of the cross sections, with the presence of more concentrated pores on the surface.
In image 1 d), a small decrease in brightness and a slightly opaque appearance can be visually appreciated, attributed to incorporating AEN into the film, but they keep their transparency. The appearance of the PA/AY/AEN is attributed to the chemical composition of the AEN, which presents a combination of resins and essential oils obtained by extraction of the characteristic spice and when combined with the film-forming solution, gives this appearance (Rodianawati, 2015). These results are in agreement with those obtained from the color analysis.
The SEM images of the surface and cross-section of the obtained PA/AY/AENs are presented in figure 1b) and e) presented a continuous, homogeneous lamellar appearance characteristic of cassava starch/glycerol/alginate. In figures 1e) and 1f), non-uniform micropores can be distinguished between the outer and inner layers of the film-forming matrix. This behavior is attributed to the hydrophobicity of the oil and its density difference with the aqueous starch solution, which may affect the stability of the film-forming solution and consequently form heterogeneous structures due to phase separation and the presence of pores (Phan et al., 2002) and may contribute to the antibacterial property of the films, considering that they facilitate the diffusion process of the essential oil from the interior of the polymeric matrix. To the surface to perform the desired action.
Physical properties
The values of moisture content, solubility, and water vapor permeability of formulated films incorporated with AEN as an antimicrobial agent are shown in table 1. The control film showed the highest moisture content (15,31 %) and water solubility (51,31 %). A significant decrease (P ≤ 0,05) in moisture content and water solubility was observed for the PA/AY-AEN films.
Incorporating AEL improves the film surface’s wetting due to its natural oily character (Istiqomah et al., 2022). These values indicate smaller free spaces in the SFP, attributed to the decrease produced by AEL of the number of hydrogen bonds with water and the formation of a denser network with better resistance (Suriyatem et al., 2018). AEN addition changed the water solubility from 51,72 % to 30,15 %, with a reduction in the diffusion of water-soluble substances due to the formation of an insoluble outer layer of starch granules (Cao et al., 2017).
|
Table 1. Moisture and solubility percentages, water vapor permeability (WVP), of films containing or not different concentrations of Orange essential oil (AEN) |
|||
|
Film |
% humidity |
% Solubility |
PVA (g/ hm2) |
|
PC |
15,31 ± 0,22a |
51,72 ± 0,21a |
52,62 ± 0,01a |
|
PA/AY/AEN -0,5 % |
13,61 ± 0,15b |
46,03 ± 0,13b |
42,28 ± 0,03b |
|
PA/AY/AEN -1,0 % |
10,72 ± 0,02c |
39,05 ± 0,12c |
38,14 ± 0,02c |
|
PA/AY/AEN -1,5 % |
8,72 ± 0,02c |
30,15 ± 0,15d |
31,27 ± 0,05d |
|
Note: Data reported are mean values ± standard deviation. The median of the same column with different letters is significantly different (Tukey: p > 0,05). |
|||
The PVA values (table 1) for all films are relatively low. The highest PVA value was the control film (52,62 g/ hm2), while the film formulated with 1,5 % AEN had the lowest PA/AY/AEN -1,5 % (31,27 g/ hm2). These values indicate to us that the hydrophobic monoterpenes present in AEN influence the hydrophobic properties of starch films and thus affect the water vapor transfer of the films, thus reducing the PVA by increasing the hydrophobicity of the formulated films (Yanwon et al., P., 2015).
The thickness of the films (table 2) ranged from 0,084 to 0,106 mm; no significant changes were presented (P < 0,05). It was observed that there is good compatibility between the SFP components, and the thickness of the PA/AY/AEN film increased by 0,106 mm, which may be associated with the solids contained in the AEN. AEL incorporation improved (p < 0,05) the tensile stress (ET) and percent elongation (%E) of PA/AY/AEN (table 2) compared to PC. This behavior is attributed to the presence of several additional functional groups, such as hydroxyl, ketone and ketone groups hydroxyl, ketone and ester groups in the AEL components, which can form stronger interactions between the components (de Oliveira et al., 2020).
Table 2. Thickness and mechanical properties of films containing or not containing different concentrations of orange essential oil (AEN) |
|||
|
Films |
Thickness (mm) |
TS (MPa) |
EB (%) |
|
PC |
0,084± 0,02NS |
5,37±0,23 a |
22,70±0,82 a |
|
PA/AY/AEN -0,5 % |
0,106± 0,01 |
8,27±0,36 b |
20,53±0,61 a |
|
PA/AY/AEN -1,0 % |
0,107± 0,03 |
11,53±0,74 c |
18,35±0,17 b |
|
PA/AY/AEN -1,5 % |
0,106± 0,01 |
15,82±0,95 d |
14,43±0,23 c |
|
Note: Data reported are mean values ± standard deviation. The median of the same column with different letters is significantly different (Tukey: p > 0,05). |
|||
Film color and opacity
Table 3 shows the results of color properties (L*. a*. b*) and opacity of PA/AY/AEN. The lightness (L*) decreased significantly (p < 0,05) as the concentration of AEN increased. The b* values increased significantly (p < 0,05) in PA/AY/AEN compared to PC; the values obtained for the* coordinate did not show significant differences, indicating a tendency to slightly yellow tones. Therefore, the color changes of the formulated films depend directly on the type and concentration of the essential oil added (Mendes et al., 2019).
Adding AEN increased opacity, which could be attributed to the fact that essential oils dispersed in the polymeric matrix increase light scattering, resulting in higher opacity of the films (Table 3). This behavior is due to the change in the refractive index of the film at the polymer interface promoted by the addition of essential oils.
|
Table 3. CIEL*a*b* coordinates and opacity contained in films with or without different concentrations of orange essential oil. (AEN) |
||||
|
Films |
L* |
a* |
b* |
Opacity |
|
PC |
87,26±0,11 a |
-1,64±0,57 a |
2,67±0,09 a |
0,40±0,06 a |
|
PA/AY/AEN -0,5 % |
85,07±0,35 b |
-1,54±0,41 a |
2,98±0,05 b |
0,52±0,04 b |
|
PA/AY/AEN -1,0 % |
83,21±0,74 b |
-1,32±0,61 a |
3,21±0,06 b |
0,72±0,01 c |
|
PA/AY/AEN -1,5 % |
80,62±0,42 c |
-1,27±0,58 a |
4,62±0,08 b |
0,89±0,02 d |
|
Note: Data reported are mean values ± standard deviation. The median of the same column with different letters is significantly different (Tukey: p > 0,05). |
||||
Essential oils dispersed in the polymer matrix promote an increase in light scattering and, consequently, in the opacity of the films. This behavior is due to the change in the film’s refractive index at the polymer interface promoted by adding essential oils (Valencia-Sullca et al., 2018).
Antimicrobial Activity
Table 4 shows the results obtained for antimicrobial activity using the disk diffusion method. PC did not show antimicrobial activity against the microorganisms tested. Inhibition halos presented in PA/AY/AEN showed higher antimicrobial activity (p < 0,05) against Staphylococcus aureus. It has been reported that the antimicrobial activity observed in AEN could be linked to the significant presence of 84 % d-limonene and 2,1 % β-myrcene main constituents of AEL. D-limonene usually exhibits antimicrobial and antiseptic activities, and β-myrcene, the second main constituent of AEN, also has antimicrobial activity (Jarine et al., 2019).
These interactions result in a gradual release of the antimicrobial compounds and ensure their action for longer than direct application ( Atarés & Chiralt, 2016 ). In addition, the incorporation of essential oils into packaging is interesting because it is an indirect method of using this natural extract in food without the need to add it as an ingredient, thus reducing undesirable sensory interferences (Calo et al., 2015).
|
Table 4. Antibacterial activity of films containing or not different concentrations of orange essential oil against Escherichia coli (ATCC 25922) and Staphylococcus aureus (ATCC 25923) |
||
|
Zone of inhibition (mm) |
||
|
Films |
E. coli |
S. aureus |
|
PC |
0,0 a |
0,0 a |
|
PA/AY/AEN -0,5 % |
10,21±1,05 b |
12,70±1,25 b |
|
PA/AY/AEN -1,0 % |
11,65±1,32 b |
14,68±1,27 b |
|
PA/AY/AEN -1,5 % |
12,25±1,40 c |
14,87±1,19 c |
|
Note: Data reported are mean values ± standard deviation. The median of the same column with different letters is significantly different (Tukey: p > 0,05). |
||
CONCLUSIONS
The present study investigated the effect of orange essential oil incorporation on the formulated films’ physical, optical, and structural properties. The main components of orange essential oil identified were 84 % d-limonene and 2,1 % β-myrcene, which exhibit antimicrobial characteristics that could contribute to extending the shelf life of packaged foods. The addition of orange essential oil evidenced improvements in decreased moisture content, solubility, and water vapor permeability values. Also, its effect as an antimicrobial agent was evidenced, presenting inhibition against E. coli and S. aureus strains, evidencing that the great positive bacteria were more sensitive to this organic compound. The results obtained indicated that the incorporation of orange essential oil was positive for antimicrobial activation and integral and film performance, indicating its potential use in food packaging.
REFERENCES
1. Atarés, L., & Chiralt, A. (2016). Essential oils as additives in biodegradable films and coatings for active food packaging. Trends in food science & technology, 48, 51- 62.
2. Aguiar, M.F. M.C.S. das Graças Fernandes da Silva, J.B. Fernandes, M.R. Forim. (2020), Evaluation of the microencapsulation of orange essential oil in biopolymers by using a spray–drying process. Sci. Rep., 10 pp. 1-11, 10.1038/s41598–020– 68823–4
3. Calo, J. R., Crandall, P. G., O’Bryan, C. A., & Ricke, S. C. (2015). Essential oils as antimicrobials in food systems–A review. Food control, 54, 111-119.
4. Chillo, S., Flores, S., (2008). Mastromatteo, M., Conte, A., Gerschenson, L., & Del Nobile.
5. M. A. Influence of glycerol and chitosan on tapioca starch-based edible film properties. Journal of Food Engineering, 88(2), 159-168
6. Jafarzadeha, S.M. Jafarib, A. Salehabadia, A.M. Nafchia, U.S.U. Kumara, H.P.S.A. Khalila (2020). Biodegradable green packaging with antimicrobial functions based on the bioactive compounds from tropical plants and their by-products. Trends in Food Science & Technology, 100, 262-277.
7. Jarine Amaral do Evangelho, Guilherme da Silva Dannenberg, Barbara Biduski, Shanise Lisie Mello el Halal, et al., (2019) Antibacterial activity, optical, mechanical, and barrier properties of corn starch films containing orange essential oil, Carbohydrate Polymers, Volume 222. https://doi.org/10.1016/j.carbpol.2019.114981.
8. Menzel, C. (2020). Improvement of starch films for food packaging through a three- principle approach: Antioxidants, cross-linking and reinforcement. Carbohydrate Polymers, 250, 116828.
9. Sadaf Nazir, Idrees Ahmed Wani, (2022). Development and characterization of an antimicrobial edible film from basil seed (Ocimum basilicum L.) mucilage and sodium alginate, Biocatalysis and Agricultural Biotechnology, Volume 44, https://doi.org/10.1016/j.bcab.2022.102450.
10. Sahraee, S. Milani, J.M. Regenstein, J.M. Kafil, H.S. (2019). Protection of foods against oxidative deterioration using edible films and coatings: a review, Food Biosci. 32 https://doi.org/10.1016/j.fbio.2019.100451.
11. Sharma, N., & Tripathi, A. (2008). Effects of Citrus sinensis (L.) Osbeck epicarp essential oil on growth and morphogenesis of Aspergillus niger (L.) Van Tieghem. Microbiological research, 163(3), 337-344.
12. Silveira Júnior, V. A.S. Prata, F.de M. Ramos, V.Silveira Júnior, A.S. Prata (2021). Physical aspects of orange essential oil–contaning particles after vacuum spray drying processing. Food Chem. X., 12 (2021), p. 100142, 10.1016/j.fochx.2021.100142.
13. Tan, W. Dong, F. Zhang, J. Zhao, X. Li, Q. Guo, Z. (2019). Physical and antioxidant properties of edible chitosan ascorbate films, J. Agric. Food Chem. 67 2530–2539. Torres, O. L., Galeano, Y. V., & Lema, M. (2021). Effect of incorporating extracts from natural pigments in alginate/starch films. Journal of Materials Research and Technology, 13, 2239-2250.
14. Valencia-Sullca. C. Vargas. M. Atarés. L. & Chiralt. A. (2018). Thermoplastic cassava starch-chitosan bilayer films containing essential oils. Food hydrocolloids. 75. 107-115.
15. Yousuf, B., S. Wu, MW Siddiqui. (2021). Incorporación de aceites esenciales o compuestos derivados de ellos en recubrimientos comestibles: efecto sobre la calidad y vida útil de productos frescos/recién cortados. Tendencias en ciencia y tecnología de los alimentos, 108, págs. 245-257, 10.1016/j.tifs.2021.01.016.
16. Zhu, Y., C. Li, H. Cui, L. Lin. (2021). Estrategias de encapsulación para mejorar las propiedades antibacterianas de los aceites esenciales en el sistema alimentario. Control de Alimentos,123 (2021), pág. 107856, 10.1016/j.foodcont.2020.107856
17. Ahmed, Md. W., Haque, Md. A., Mohibbullah, Md., Khan, Md. S. I., islam, M. A., Mondal, Md. H. T., & Ahmmed, R. (2022). A review on active packaging for quality and safety of foods: Current trends, applications, prospects and challenges. Food Packaging and Shelf Life, 33, 100913. https://doi.org/10.1016/j.fpsl.2022.100913
18. Alves, J., Gaspar, P. D., Lima, T. M., & Silva, P. D. (2023). What is the role of active packaging in the future of food sustainability? A systematic review. Journal of the Science of Food and Agriculture, 103(3), 1004–1020. https://doi.org/10.1002/jsfa.11880
19. Deshmukh, R. K., Hakim, L., & Gaikwad, K. K. (2023). Active Packaging Materials. Current Food Science and Technology Reports, 1(2), 123–132. https://doi.org/10.1007/s43555-023-00004-6
20. Farousha, K., Tham, P. E., Chew, K. W., Amornraksa, S., & Show, P. L. (2023). The Future of Food Preservation: Active Packaging with Controlled Release Systems. E3S Web of Conferences, 428, 02009. https://doi.org/10.1051/e3sconf/202342802009
21. Gaikwad, K. K., Singh, S., & Negi, Y. S. (2020). Ethylene scavengers for active packaging of fresh food produce. Environmental Chemistry Letters, 18(2), 269–284. https://doi.org/10.1007/s10311-019-00938-1
22. Jacinto-Valderrama, R. A., Andrade, C. T., Pateiro, M., Lorenzo, J. M., & Conte-Junior, C. A. (2023). Recent Trends in Active Packaging Using Nanotechnology to Inhibit Oxidation and Microbiological Growth in Muscle Foods. Foods, 12(19), Article 19. https://doi.org/10.3390/foods12193662
23. Jafarzadeh, S., Hadidi, M., Forough, M., Nafchi, A. M., & Mousavi Khaneghah, A. (2023). The control of fungi and mycotoxins by food active packaging: A review. Critical Reviews in Food Science and Nutrition, 63(23), 6393–6411. https://doi.org/10.1080/10408398.2022.2031099
24. Jha, P. (2020). Effect of grapefruit seed extract ratios on functional properties of corn starch-chitosan bionanocomposite films for active packaging. International Journal of Biological Macromolecules, 163, 1546–1556. https://doi.org/10.1016/j.ijbiomac.2020.07.251
25. Just, D. R., & Goddard, J. M. (2023). Behavioral framing and consumer acceptance of new food technologies: Factors influencing consumer demand for active packaging. Agribusiness, 39(1), 3–27. https://doi.org/10.1002/agr.21778
26. Kuai, L., Liu, F., Chiou, B.-S., Avena-Bustillos, R. J., McHugh, T. H., & Zhong, F. (2021). Controlled release of antioxidants from active food packaging: A review. Food Hydrocolloids, 120, 106992. https://doi.org/10.1016/j.foodhyd.2021.106992
27. Kumar, S., & Thakur, K. S. (2020). Active packaging technology to retain storage quality of pear cv. “Bartlett” during shelf-life periods under ambient holding after periodic cold storage. Packaging Technology and Science, 33(7), 239–254. https://doi.org/10.1002/pts.2501
28. López-Gómez, A., Navarro-Martínez, A., Garre, A., Iguaz, A., & Martínez-Hernández, G. B. (2023). The Potential of Essential Oils from Active Packaging to Reduce Ethylene Biosynthesis in Plant Products. Part 2: Fruits (Blueberries and Blackberries). Plants, 12(19), Article 19. https://doi.org/10.3390/plants12193418
29. Monção, É. da C., Grisi, C. V. B., de Moura Fernandes, J., Souza, P. S., & de Souza, A. L. (2022). Active packaging for lipid foods and development challenges for marketing. Food Bioscience, 45, 101370. https://doi.org/10.1016/j.fbio.2021.101370
30. Nimitkeatkai, H., Techavuthiporn, C., Boonyaritthongchai, P., & Supapvanich, S. (2022). Commercial active packaging maintaining physicochemical qualities of carambola fruit during cold storage. Food Packaging and Shelf Life, 32, 100834. https://doi.org/10.1016/j.fpsl.2022.100834
31. Pascuta, M. S., & Vodnar, D. C. (2022). Nanocarriers for Sustainable Active Packaging: An Overview during and Post COVID-19. Coatings, 12(1), Article 1. https://doi.org/10.3390/coatings12010102
32. Qian, M., Liu, D., Zhang, X., Yin, Z., Ismail, B. B., Ye, X., & Guo, M. (2021). A review of active packaging in bakery products: Applications and future trends. Trends in Food Science & Technology, 114, 459–471. https://doi.org/10.1016/j.tifs.2021.06.009
33. Roopa, H., Panghal, A., Kumari, A., Chhikara, N., Sehgal, E., & Rawat, K. (2023). Active Packaging in Food Industry. In Novel Technologies in Food Science (pp. 375–404). John Wiley & Sons, Ltd. https://doi.org/10.1002/9781119776376.ch10
34. R. Westlake, J., W. Tran, M., Jiang, Y., Zhang, X., D. Burrows, A., & Xie, M. (2023). Biodegradable biopolymers for active packaging: Demand, development and directions. Sustainable Food Technology, 1(1), 50–72. https://doi.org/10.1039/D2FB00004K
35. Singh, A. K., Ramakanth, D., Kumar, A., Lee, Y. S., & Gaikwad, K. K. (2021). Active packaging technologies for clean label food products: A review. Journal of Food Measurement and Characterization, 15(5), 4314–4324. https://doi.org/10.1007/s11694-021-01024-3
36. Sirait, M. S., Warsiki, E., & Setyaningsih, D. (2021). Potential of red fruit oil (Pandanus conoideus Lam.) as an antioxidant active packaging: A review. IOP Conference Series: Earth and Environmental Science, 749(1), 012008. https://doi.org/10.1088/1755-1315/749/1/012008
37. Soltani Firouz, M., Mohi-Alden, K., & Omid, M. (2021). A critical review on intelligent and active packaging in the food industry: Research and development. Food Research International, 141, 110113. https://doi.org/10.1016/j.foodres.2021.110113
38. Su, J., Luo, Y., Cao, Z., Wang, X., & Ge, X. (2024). Advances in loquat post-harvest preservation and the application of nanotechnology for its active packaging. Journal of Food Process Engineering, 47(1), e14507. https://doi.org/10.1111/jfpe.14507
39. Thirupathi Vasuki, M., Kadirvel, V., & Pejavara Narayana, G. (2023). Smart packaging—An overview of concepts and applications in various food industries. Food Bioengineering, 2(1), 25–41. https://doi.org/10.1002/fbe2.12038
40. Umair, M., Sultana, T., Xun, S., Jabbar, S., Riaz Rajoka, M. S., Albahi, A., Abid, M., Ranjha, M. M. A. N., El-Seedi, H. R., Xie, F., Khan, K. ur R., Liqing, Z., & Zhendan, H. (2023). Advances in the application of functional nanomaterial and cold plasma for the fresh-keeping active packaging of meat. Food Science & Nutrition, 11(10), 5753–5772. https://doi.org/10.1002/fsn3.3540
41. Westlake, J. R., Tran, M. W., Jiang, Y., Zhang, X., Burrows, A. D., & Xie, M. (2022). Biodegradable Active Packaging with Controlled Release: Principles, Progress, and Prospects. ACS Food Science & Technology, 2(8), 1166–1183. https://doi.org/10.1021/acsfoodscitech.2c00070
FINANCING
None.
CONFLICT OF INTEREST
None.
AUTHORSHIP CONTRIBUTION
Conceptualization: Olga Lucia Torres Vargas, Iván Andrés Rodríguez Agredo.
Writing - initial draft: Olga Lucia Torres Vargas, Iván Andrés Rodríguez Agredo.
Writing -revision and editing: Olga Lucia Torres Vargas, Iván Andrés Rodríguez Agredo.