doi: 10.62486/agmu202321
ORIGINAL
Analysis of antibody titers according to anti-SARS-CoV-2 vaccination schedules, assessed through protein S, discriminated according to history of Covid-19 disease, comorbidities, age and sex in San Lorenzo city
Análisis de los títulos de anticuerpos según esquemas de vacunación anti-SARS-CoV-2, valorados a través de la proteína S, discriminados según antecedente de enfermedad Covid-19, comorbilidades, edad y sexo en Ciudad de San Lorenzo
1Universidad Abierta Interamericana. Santa Fe, Argentina.
Cite as: González Y. Analysis of antibody titers according to anti-SARS-CoV-2 vaccination schedules, assessed through protein S, discriminated according to history of Covid-19 disease, comorbidities, age and sex in San Lorenzo city. Multidisciplinar (Montevideo). 2023; 1:21. https://doi.org/10.62486/agmu202321
Submitted: 10-07-2023 Revised: 18-10-2023 Accepted: 24-12-2023 Published: 25-12-2023
Editor:
Telmo Raúl Aveiro-Róbalo ![]()
ABSTRACT
The present study analyzes the humoral immune response in 319 inhabitants of San Lorenzo, Argentina, vaccinated with two doses of different anti-SARS-CoV-2 vaccines. The results show that almost all participants developed Anti-S antibodies, with the Sinopharm vaccine being less effective compared to AstraZeneca, Sputnik V and Moderna. The population was divided according to variables such as sex, age, history of Covid-19 and comorbidities, and it was evident that both people with a history of the disease and those with comorbidities had elevated antibody levels. No significant differences in immunogenicity were found with respect to sex or age, confirming a high response in older adults. The main limitation was a fixed cut-off point of 250 U/ml, which prevented detailed comparisons between schemes. This study suggests the effectiveness of all vaccination schedules studied and underlines the need for future research on the durability of the immune response.
Keywords: Covid-19; SARS-CoV-2; Protein S; Vaccines; Immune Response; Antibodies.
RESUMEN
El presente estudio analiza la respuesta inmunitaria humoral en 319 habitantes de San Lorenzo, Argentina, vacunados con dos dosis de distintas vacunas anti-SARS-CoV-2. Los resultados muestran que casi todos los participantes desarrollaron anticuerpos Anti-S, siendo menos efectiva la vacuna Sinopharm en comparación con AstraZeneca, Sputnik V y Moderna. La población fue dividida en función de variables como sexo, edad, antecedentes de Covid-19 y comorbilidades, y se evidenció que tanto personas con antecedentes de la enfermedad como aquellas con comorbilidades presentaron niveles de anticuerpos elevados. No se encontraron diferencias significativas en la inmunogenicidad respecto al sexo ni la edad, confirmando una respuesta elevada en adultos mayores. La principal limitación fue un punto de corte fijo de 250 U/ml, que impidió comparaciones detalladas entre esquemas. Este estudio sugiere la efectividad de todos los esquemas de vacunación estudiados y subraya la necesidad de investigaciones futuras sobre la durabilidad de la respuesta inmunitaria.
Palabras clave: Covid-19; SARS-CoV-2; Proteína S; Vacunas; Respuesta Inmune; Anticuerpos.
INTRODUCTION
On December 29, 2019, in Wuhan, a Chinese city with a population of 11 million, the first four cases of "pneumonia of unknown origin" were reported, all from workers at the Huanan Seafood Wholesale Market. It was soon established that this severe acute respiratory syndrome was caused by a new coronavirus called SARS-CoV-2.(1) Rapid and effective transmission led to its global spread. On March 11, 2020, the World Health Organization (hereinafter WHO) declared it a pandemic, keeping much of the world's population under recommended social distancing and/or lockdown measures to date.(2)
According to information published by the Johns Hopkins University Center for Systems Science and Engineering, at the time of writing this review, there are more than 676 million confirmed cases worldwide and more than 6,6 million deaths recorded.(3) An analysis of 1,3 million cases in the United States showed that 14 % of those infected required hospitalization, 2 % of them in intensive care, and a mortality rate of 5 %. The same analysis found that patients with comorbidities were six times more likely to be hospitalized and 12 times more likely to die than those without comorbidities.(4)
According to the Ministry of Health, on March 3, 2020, the first positive case (+) of this new disease that united the world in this major epidemiological emergency was confirmed in Argentina.(5)
On December 23, 2020, the Ministry of Health of the Argentine Republic stated that 14,9 % of confirmed cases were in people over 60 years of age, but at the same time, 82,7 % of deaths were in this group. In turn, the fatality rate by age group in Argentina showed a substantial increase above the age of 70, which is evident when observing that for all age groups, the average fatality rate is 2,7 %, while for the group of adults aged 70 and over, this figure rises to 18,3 % and reaches 30,1 % in the group aged 80 and over.(6)
Due to the epidemiological situation that alerted the world, it was imperative to develop a vaccine to reduce the mortality rate, the length of hospital stays, and the infection rate worldwide.
The S protein on the viral surface has been identified as the optimal antigen for vaccine development.(2) The Ministry of Health, as the governing body of the health system, has designed the "Strategic Plan for COVID-19 Vaccination in Argentina,"(6) which began to be implemented in January 2021.
As local background information on the study and research into the production of antibodies from the administration of vaccines, epidemiological studies have been carried out at the Vélez Sarsfield Hospital in the city of Buenos Aires and the Dr. H. Noel Sbarra in the town of La Plata. Both studies evaluated the response to the Sputnik V regimen, as this vaccine was the first to arrive in Argentina and, therefore, to be administered to healthcare workers, resulting in seroconversion in more than 90 % of the study population.(7,8)
Reports on the efficacy and safety of this vaccine have been published in Phase 3 clinical trials.(9) However, due to the urgent conditions surrounding the development and approval of vaccines in general, it is of scientific interest to evaluate the immune response to this and other vaccines in our population.
To expand studies on the relevance of this topic and due to the diversity of vaccination platforms approved to date, we have chosen to analyze the effectiveness of Anti-S antibody production in residents of the city of San Lorenzo (province of Santa Fe) who have completed the vaccination schedule (two doses), taking into account variables such as age, sex, history of COVID-19 disease, and comorbidities. In summary, and taking into account the genetic, pathological, and age diversity of these individuals, this study will attempt to answer the following question: What are the results obtained in the titration of anti-SARS-CoV-2 antibodies with the inoculation of the different vaccination schedules applied during the period October-November 2021 to the population over 18 years of age residing in the city of San Lorenzo?
METHOD
The study was quantitative, descriptive, observational, and cross-sectional. Data collection was retrospective. The research was conducted during the second half of 2022.
This study was based on data from people who voluntarily participated in the DetectAR Campaign, organized by the Municipality of San Lorenzo, during October and November 2021. The DetectAR Campaign is a public policy proposed by the national government and carried out by health professionals and administrators from each province and/or municipality. In this case, it was carried out by doctors, nurses, physical therapists, psychologists, paramedics, and administrative staff from the Ministry of Health and Environment. Each Territorial Device was coordinated to be carried out in vulnerable neighborhoods of the city as a strategy based on Primary Health Care to achieve equitable access. The Press Office of the Municipality of San Lorenzo publicized the event, which took place on Mondays during the months above.
The study population consisted of 346 people who voluntarily attended the DetectAR Campaign organized by the Municipality of San Lorenzo during October and November 2021. A sample that included people of both sexes over 18 years of age with a complete vaccination schedule at the time of sampling was selected. Those who did not present a vaccination certificate with the two doses reported on the sampling date, who had been inoculated with more than two doses or a booster (three doses), and who were not residents of the locality were excluded.
A non-probabilistic sampling method was used for convenience. The sample analyzed consisted of individuals who had attended the DetectAR Campaign in a timely manner with their respective vaccination cards certifying the two doses administered and who were also residents of San Lorenzo.
The database belonging to the Ministry of Health and Environment of the Municipality of San Lorenzo was used. This database contains the results of the Anti-Spike tests carried out in October and November 2021. The data provided by this institution for this research was obtained through the collection of biological samples by health personnel during the DetectAR program and their subsequent analysis at the Center for Public Health Technology (hereinafter CTSP) of the Faculty of Biochemical and Pharmaceutical Sciences of the National University of Rosario.
Samples were obtained from the population by blood extraction (5 ml of peripheral blood by venipuncture), and the determination of IgG antibodies against the receptor binding domain of the S1 subunit of the spike protein (Spike or S) of the SARS-CoV-2 virus in serum was carried out at the CTSP in Rosario. Samples that met the acceptance criteria were analyzed using the Elecsys® Anti-SARSCoV-2 S test, an immunoassay for the quantitative in vitro detection of antibodies against the receptor binding domain (RBD) of the SARS-CoV-2 spike protein in human serum and plasma. The measurement was performed on the cobas® e411 automated analyzer using the immunofluorescence method. The results were expressed in arbitrary units (U)/ml, and the cutoff point was 0,8 U/ml—those with values less than 0,8 U/ml were considered harmful. Results greater than 0,9 U/ml were considered positive. However, positive results were discriminated up to 250 U/mL, as those exceeding this value were expressed as greater than 250 U/mL. A study form was completed, including information on the National Identity Document (DNI), date of birth, sex, comorbidities, date of vaccination, and history of COVID-19.
Participants were informed of their SARS-CoV-2 IgG antibody titer through a written protocol within 15 days after sample collection.
Variables such as sex, age, history of COVID-19 disease, and comorbidities were analyzed according to data provided by the San Lorenzo Municipality Health and Environment Secretariat, which were recorded in Microsoft Excel spreadsheets. Tables were created to visualize the different variables to be described, which allowed the effects on antibody titers defined by the Elecsys® Anti-SARSCoV-2 S test to be calculated according to the variables. Microsoft Excel also used central tendency measures (mean and median) and dispersion measures (standard deviation, interquartile range).
The study complied with the ethical principles of Law 26,529. “Rights of Patients in their Relationship with Health Professionals and Institutions,” for using patient data for research purposes. This research ensures the confidentiality and anonymity of the individuals involved, as promoted by the Declaration of Helsinki and the Personal Data Protection Act, Law 25,326.(10,11,12)
RESULTS
Cohort characteristics
The final study comprised 319 subjects: 228 (71,5 %) women and 91 (28,5 %) men. The sample's age was 52 ± 14 years (table 1).
According to the vaccination schedule received, the cohort could be divided into four groups: a first group consisting of 87 people who received AZ+AZ, a second group of 80 people who received SINO+SINO, a third group of 44 people vaccinated with SP+MO, and a final group of 108 people with the SP+SP schedule (table 2).
The record of participants regarding whether or not they had had SARS-CoV-2 disease allowed them to be divided into two groups: individuals who did not have SARS-CoV-2 disease, or at least were not aware of it, and those who said they had had it, with a test to certify it. There were 48 people (15,1 %) in the SARS-CoV-2 group and 271 (84,9 %) in the group without previous SARS-CoV-2 disease (table 3).
Of the total participants, 89,3 % had no associated comorbidities. The remaining percentage, represented by 34 participants, reported having some pathological history (table 4).
|
Table 1. Cohort characteristics by age and sex |
||
|
Sex |
≥65 years old |
N (%) |
|
Feminine |
No |
182 (79,8) |
|
Yes |
46 (20,1) |
|
|
Totally feminine |
|
228 (71,5) |
|
Male |
No |
65 (71,4) |
|
Yes |
26 (28,5) |
|
|
Totally masculine |
|
91 (28,5) |
|
Total |
319 |
|
|
Table 2. Characteristics of the cohort according to the vaccination schedule received |
|
|
Name |
N (%) |
|
AZ +AZ |
87 (27,2) |
|
SINO + SINO |
80 (25,1) |
|
SP + MO |
44 (13,1) |
|
SP + SP |
108 (33,8) |
|
Sum total |
319 |
|
Table 3. Characteristics of the cohort according to COVID-19 history |
|
|
Background Covid-19 |
N (%) |
|
NO |
271 (84,9) |
|
YES |
48 (15,1) |
|
Sum total |
319 |
|
Table 4. Characteristics of the cohort according to history of comorbidities |
|
|
Comorbidities |
N |
|
Rheumatoid arthritis |
1 |
|
Bronchial asthma |
1 |
|
Type 2 diabetes mellitus |
7 |
|
Breast cancer |
1 |
|
Pulmonary emphysema |
1 |
|
Dyslipidemia + Obesity |
6 |
|
Hypothyroidism |
6 |
|
Acute myocardial infarction |
1 |
|
High blood pressure |
17 |
|
Arrhythmia (pacemaker use) |
1 |
|
Melanoma |
1 |
|
Psoriasis |
1 |
|
Comorbidities |
34 |
|
No comorbidities |
285 |
Notably, almost all participants developed antibodies against the RBD domain, except two (0,63 %) vaccinated with the SINO+SINO regimen.
The median antibody titer against the RBD domain was 250 U/mL (250-233,4), and the minimum value was 1 U/mL. In 237 participants (74,3 %), levels ≥ 250 U/mL were found, the maximum level of antibodies reported by the test.
The antibody titer with the SP + SP regimen was found to have a median of 217,5 U/mL (250 and 250) and a mean of 217,5 ± 70,8 U/mL. Similarly, the AZ+AZ regimen confirmed a median of 250 U/mL (250 and 250) and a mean of 230,2 ± 55,9 U/mL. Similarly, despite having a lower n, the SP+MO regimen yielded the same results for its median and interquartile index (Me 250 U/mL, 250-250) and a mean of 250 ± 0 U/mL. However, the regimen with the lowest antibody titer compared to the others was SINO+SINO, with a median of 169,5 U/mL (250-33,8) and a mean of 142,2 ± 105,3 U/mL.
The data analyzed above showed that immunization with AZ, SP+MO, and SP produced a positive and similar humoral response. However, when the aforementioned regimens were compared with the SINO regimen, the values were lower. In other words, the humoral response analyzed with the Sinopharm vaccine was, on average, lower than with immunization with AZ, SP+MO, and SP.
On the other hand, females had a mean of 203,8 ± 83,7 U/mL, while males had a mean of 213,5 ± 78,9 U/mL. The data obtained from the classification by sex showed similar results in quantifying antibody titers.
The median age of individuals ≥ 65 years at sampling was calculated for the age variable, which was 250 U/mL (250-250). The antibody titer in subjects ≤64 years was 250 U/mL (250-195,2). These values indicate no differences in the humoral response between the two groups.
Note for patients with a history of medical conditions and/or comorbidities
Table 5 shows that 10,6 % (n: 34 participants) of the total cohort had a history of disease or comorbidities.
Of the total 10,6 %, 76,5 % reached the maximum antibody titers quantifiable by this method, i.e., 26 participants reached titers greater than 250 U/ml, while 8 participants reached lower values.
|
Table 5. Classification of participants according to comorbidity, vaccination schedule, and antibody titers |
||
|
Patient |
Comorbidities |
Diagram - Anti-S Result |
|
Pacient 1 |
Hypothyroidism |
SP+SP >250,0 |
|
Pacient 2 |
Type 2 diabetes mellitus, Breast cancer |
SINO+SINO >250,0 |
|
Pacient 3 |
Hypothyroidism |
SP+SP >250,0 |
|
Pacient 4 |
Type 2 diabetes mellitus |
SP+SP >250,0 |
|
Pacient 5 |
High blood pressure |
SP+SP >250,0 |
|
Pacient 6 |
Obesity |
SP+SP >250,0 |
|
Pacient 7 |
Rheumatoid arthritis |
AZ+AZ >250,0 |
|
Pacient 8 |
High blood pressure |
AZ+AZ >250,0 |
|
Pacient 9 |
Pulmonary emphysema, High blood pressure, Dyslipidemia |
SP+SP >250,0 |
|
Pacient 10 |
Acute myocardial infarction, dyslipidemia, high blood pressure |
SP+SP >250,0 |
|
Pacient 11 |
High blood pressure, arrhythmia (pacemaker use) |
SP+MO >250,0 |
|
Pacient 12 |
Dyslipidemia + obesity |
SP+SP >250,0 |
|
Pacient 13 |
High blood pressure |
SP+MO >250,0 |
|
Pacient 14 |
High blood pressure |
SP+MO >250,0 |
|
Pacient 15 |
Psoriasis |
AZ+AZ >250,0 |
|
Pacient 16 |
Type 2 diabetes mellitus |
SINO+SINO >250,0 |
|
Pacient 17 |
High blood pressure |
SP+MO >250,0 |
|
Pacient 18 |
Melanoma |
SINO+SINO >250,0 |
|
Pacient 19 |
Bronchial asthma |
AZ+AZ >250,0 |
|
Pacient 20 |
High blood pressure |
SINO+SINO >250,0 |
|
Pacient 21 |
Type 2 diabetes mellitus |
AZ+AZ >250,0 |
|
Pacient 22 |
High blood pressure |
SP+MO >250,0 |
|
Pacient 23 |
High blood pressure |
SP+MO >250,0 |
|
Pacient 24 |
High blood pressure |
SP+MO >250,0 |
|
Pacient 25 |
Type 2 diabetes mellitus |
SP+MO >250,0 |
|
Pacient 26 |
Hypothyroidism |
SP+SP >250,0 |
|
Pacient 27 |
Hypothyroidism |
SINO+SINO 235,8 |
|
Pacient 28 |
Hypothyroidism |
SINO+SINO 61,14 |
|
Pacient 29 |
High blood pressure, Type 2 diabetes mellitus |
SINO+SINO 59,69 |
|
Pacient 30 |
High blood pressure |
AZ+AZ 53,23 |
|
Pacient 31 |
Hypothyroidism |
SINO+SINO 39,73 |
|
Pacient 32 |
High blood pressure |
SINO+SINO 36,75 |
|
Pacient 33 |
High blood pressure |
SP+SP 23,56 |
|
Pacient 34 |
High blood pressure |
SINO+SINO 11,51 |
Figure 1 shows that of the 8 (23,5 %) participants who did not achieve maximum antibody titers, 6 had received the SINO+SINO regimen.
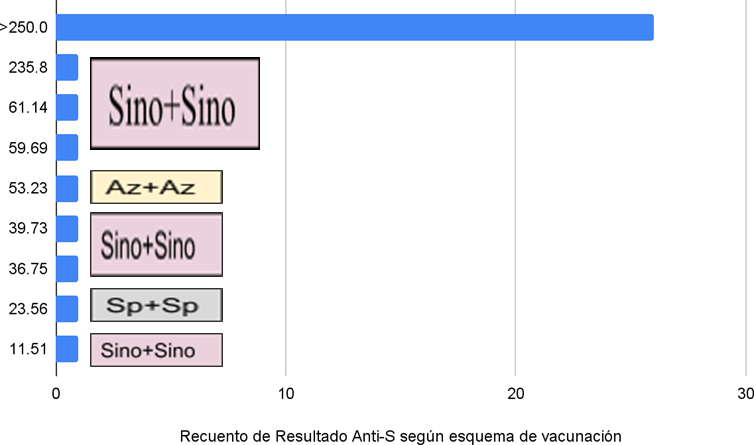
Figure 1. Count of Anti-S results below 250 U/ml in people with comorbidities according to vaccination regimen
Note for patients with a history of COVID-19
Of the patients who tested positive for Anti-S antibody titers, 15,1 % reported having previously had COVID-19, while 84,8 % reported no contact with the virus (table 6).
|
Table 6. Distribution of patients according to history (+) or (-) of COVID-19 disease |
||
|
|
Covid-19 background (+) n (%) |
Covid-19 background (-) n (%) |
|
Anti-S > 0,8 U/ml |
48 (15,1) |
269 (84,8) |
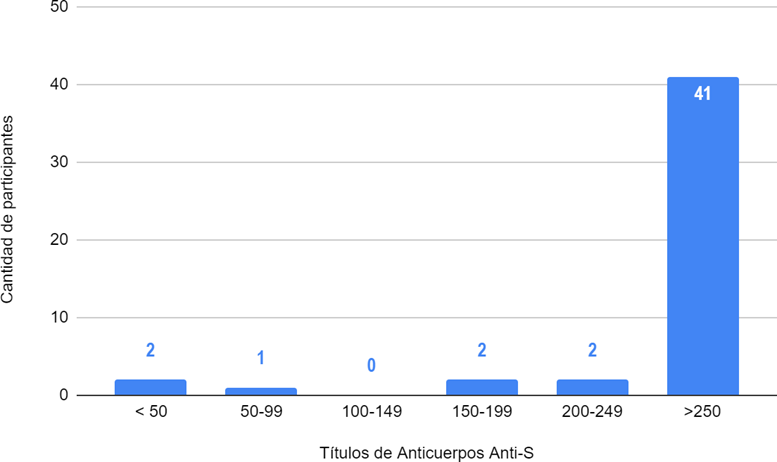
Figure 2. Distribution of antibody titers against the receptor-binding domain of the S protein in U/ml in participants with a history of COVID-19. (+)
Legend: n=%: 41=85,4 %; 2=4,1 %; 2=4,1 %; 0=0; 1=2 %; 2=4,1 %.
As shown in Graph 2, 85,4 % of participants with previous disease (41 participants) developed titers greater than 250 U/ml. The rest of the participants developed lower titers.
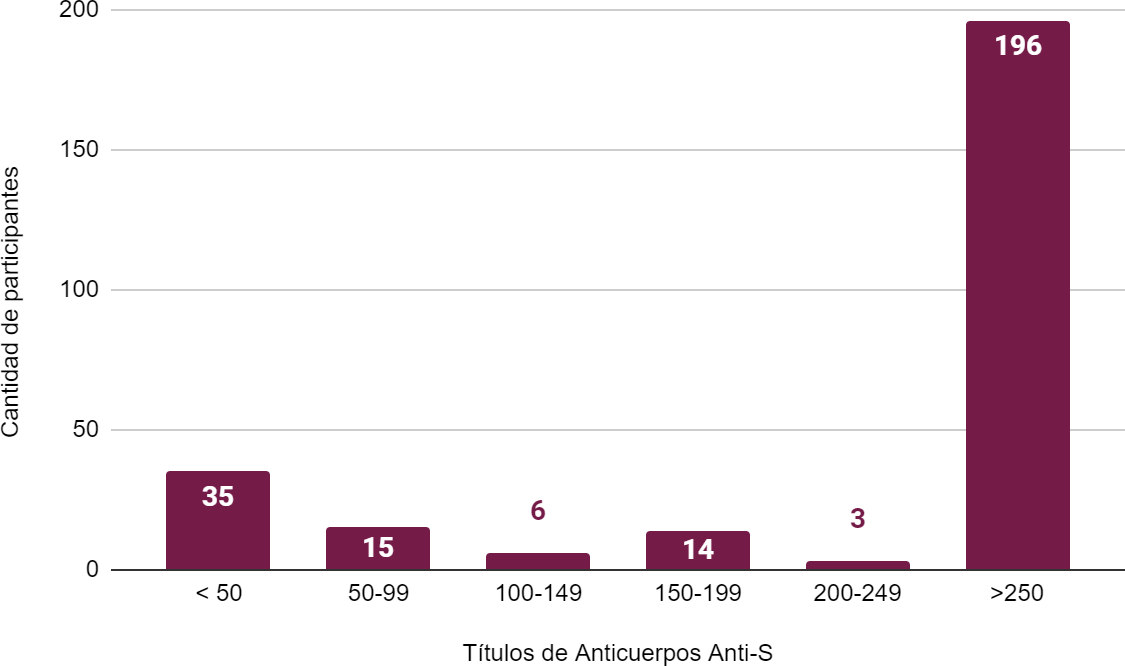
Figure 3. Distribution of antibody titers against the receptor-binding domain of the S protein in U/ml in participants with no history of COVID-19 disease
Legend: n=%: 196=72,8 %; 3=1,1 %; 14=5,2 %; 6=2,2 %; 15=5,5 %; 35=13 %.
Figure 3 shows that 72,8 % of participants who denied having had the disease (196 participants) developed titers greater than 250 U/ml. Thirteen percent (35 participants) had titers less than 50 U/ml.
DISCUSSION
Given that vaccines have eradicated diseases throughout history, there was no doubt that the creation of a SARS-CoV-2 vaccine to reduce transmissibility, morbidity, and mortality in the global population was imminent.
In this study, we analyzed the humoral immune response in a cohort of 319 people vaccinated with two doses of the following SARS-CoV-2 vaccines: SP+SP, AZ+AZ, SINO+SINO, and SP+MO.
Our study showed the presence of anti-S antibodies in almost all participants except two with the SINO+SINO regimen. This corroborates the data evaluated in other studies and confirms adequate immunogenicity in the humoral response.
Our data are consistent with the clinical trial published by Xia et al., who found adequate immune responses in a population of 143 people vaccinated with the Sinopharm regimen.(13) The clinical analysis of 168 people belonging to the Peruvian health system was published with the same regimen and similar results.(14)
Immunization with AstraZeneca, Sputnik V, and Moderna increased the humoral response studied the most compared to those immunized with Sinopharm.
The mean antibody titers the SP+SP and SP+MO regimens produced exceeded 200 U/mL and 250 U/mL, respectively. In our country, trials conducted in the provinces of Buenos Aires and La Rioja with the SP+SP regimen during 2021 (7,30),(7,15) concluded the same result, with no national references on the SP+MO regimen found at the time of writing this research.
The results of the AZ+AZ regimen exceeded 230 U/mL in the vast majority of participants; this value is consistent with the statistical data published in an article in the scientific journal The Lancet, which compared the results of four randomized controlled clinical trials in the United Kingdom, South Africa, and Brazil.(16)
Although the effectiveness was positive in all regimens, the analysis revealed a slight difference in the elevation of anti-spike antibody titers in the Sinopharm regimen, which was lower than in the other regimens.
Regarding gender, the statistics in this study show no difference in the efficacy of vaccines for this variable. However, Soto et al. suggest that females' humoral response was higher.(14) This result may be related to the article published by Takahashi et al., who postulated that the T cell-mediated immune response appears to be more intense in women, which also correlates with the fact that the severity of COVID-19 is lower in females.(17)
About the age of the participants, there was no decrease in immunogenicity in those ≥ 65 years of age. Furthermore, it is essential to note that a study with twice as many participants (660 candidates) in the province of Buenos Aires yielded the same result.(7)
All participants with comorbidities developed a humoral response to SARS-CoV-2 vaccination. A total of 23,5 %, corresponding to 8 participants, did not exceed 250 U/ml; six were vaccinated with SINO+SINO. However, studies with a larger population are needed to determine whether significant differences exist.
Nevertheless, this study's sample size is consistent with other research (7,8) which indicates that having previously had SARS-CoV-2 disease generates immune memory and that exposure to the vaccine produces higher titers than in those without previous infection.
In the future, evaluating the persistence of antibody titers generated by vaccination schedules over time would be helpful. Analyzing the cellular immunity conferred by these vaccination schedules would also be relevant. However, it is known that this requires costly equipment that is only available in a few places in Argentina.
CONCLUSIONS
Our data show that all vaccination schedules were immunogenic. Anti-S antibody titers were higher in people with previous COVID-19 infection than those without the disease. Subjects with comorbidities had higher anti-S antibody titers in response to inoculation, as did healthy subjects. The adult population aged ≥ 65 had a high Anti-S humoral response, as did the rest. There is no difference in vaccine efficacy for the gender variable.
Limitations
Our study has the limitation of having a fixed cutoff point (250 U/ml). This prevented comparisons between the various vaccination schedules.
REFERENCES
1. Li Q, Guan X, Wu P, Wang X, Zhou L, Tong Y, et al. Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus–Infected Pneumonia. N Engl J Med 2020;382:1199-207. https://doi.org/10.1056/NEJMoa2001316.
2. Ibáñez Guelfenbein C, Torres Torretti JP, Santolaya De Pablo ME. Vacunas SARS CoV-2, estudios en fase III. Rev Chil Infectol 2021;38:88-98. https://doi.org/10.4067/S0716-10182021000100088.
3. Johns Hopkins University. COVID-19 Dashboard. Johns Hopkins Univ 2023. https://coronavirus.jhu.edu/map.html.
4. Ashraf O, Virani A, Cheema T. COVID-19: An Update on the Epidemiological, Clinical, Preventive, and Therapeutic Management of 2019 Novel Coronavirus Disease. Crit Care Nurs Q 2021;44:128-37. https://doi.org/10.1097/CNQ.0000000000000346.
5. Argentina.gob.ar. Salud confirma el primer caso de coronavirus en el país. Argentina.gob.ar 2020. https://www.argentina.gob.ar/noticias/salud-confirma-el-primer-caso-de-coronavirus-en-el-pais.
6. Honorable Congreso de la Nación Argentina. Plan estratégico para la vacunación contra la Covid-19 en la República Argentina. 2020.
7. Rovere P, Laurelli A, Díaz A, Dabusti G, Valdez P. Seroprevalencia de anticuerpos anti S1 SARS-COV-2 en trabajadores vacunados con Sputnik V en un hospital público de la ciudad de Buenos Aires. Med BAires 2021;81:895-901.
8. Prado A, Salas Cris C, Lopez de Armentia R, Vélez A. Evaluación de la respuesta humoral frente a la vacunación Covid-19 del personal de salud del Hospital Sbarra. Sbarra Científica 2021;3.
9. Logunov DY, Dolzhikova IV, Shcheblyakov DV, Tukhvatulin AI, Zubkova OV, Dzharullaeva AS, et al. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia. The Lancet 2021;397:671-81. https://doi.org/10.1016/S0140-6736(21)00234-8.
10. Senado y Cámara de Diputados de la Nación Argentina. Derechos del Paciente en su Relación con los Profesionales e Instituciones de la Salud. 2009.
11. Senado y Cámara de Diputados de la Nación Argentina. Ley de Protección de los Datos Personales. 2000.
12. World Medical Association. Declaración de Helsinki: Principios éticos para las investigaciones médicas en participantes humanos. World Med Assoc 2024. https://www.wma.net/es/policies-post/declaracion-de-helsinki-de-la-amm-principios-eticos-para-las-investigaciones-medicas-en-seres-humanos/ (accedido 31 de octubre de 2024).
13. Xia S, Zhang Y, Wang Y, Wang H, Yang Y, Gao GF. Safety and immunogenicity of an inactivated COVID-19 vaccine, BBIBP-CorV, in people younger than 18 years: a randomised, double-blind, controlled, phase 1/2 trial. Lancet Infect Dis 2022;22:196-208. https://doi.org/10.1016/S1473-3099(21)00462-X.
14. Soto A, M C-RF, Pareja-Medina M, Fernandez-Navarro M, Altamirano-Cáceres K, Sierra Chávez E. Evaluation of the humoral response induced by BBIBP-CorV vaccine by determining neutralizing antibodies in peruvian healthcare personnel. Rev Peru Med Exp Salud Publica 2021;38:493-500. https://doi.org/10.17843/rpmesp.2021.384.9244.
15. Ridao F. Estudio clínico de evaluación humoral con el empleo de la vacuna Sputnik en La Rioja: informe parcial. La Rioja, Argentina: Centro de Investigación en Medicina Traslacional (CIMT) - Ministerio de Salud Pública de La Rioja; 2021.
16. Knoll MD, Wonodi C. Oxford–AstraZeneca COVID-19 vaccine efficacy. The Lancet 2021;397:72-4. https://doi.org/10.1016/S0140-6736(20)32623-4.
17. Takahashi T, Ellingson MK, Wong P, Israelow B, Lucas C, Klein J. Sex differences in immune responses that underlie COVID-19 disease outcomes. Nature 2020;588:315-20. https://doi.org/10.1038/s41586-020-2700-3.
FINANCING
The authors did not receive funding for the development of this research.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHOR CONTRIBUTION
Conceptualization: Yasmin González.
Data curation: Yasmin González.
Formal analysis: Yasmin González.
Research: Yasmin González.
Methodology: Yasmin González.
Project management: Yasmin González.
Resources: Yasmin González.
Software: Yasmin González.
Supervision: Yasmin González.
Validation: Yasmin González.
Visualization: Yasmin González.
Writing – original draft: Yasmin González.
Writing – review and editing: Yasmin González.
ANNEXES
List of abbreviations
· Older adults: people over 65 years of age
· ANMAT: National Administration of Medicines, Food and Medical Technology
· AZ: AstraZeneca
· DetectAR: Strategic Testing Device for Coronavirus in Argentina
· MO: Moderna
· NomiVac: Federal Nominal Vaccination Registry
· WHO: World Health Organization
· SARS-CoV-2: Severe Acute Respiratory Syndrome Coronavirus-2
· SINO: Sinopharm
· SP: Sputnik V
· SPIKE or S Protein (S protein): spike glycoprotein or spike protein
Criteria for prioritizing people for vaccination
Risk due to exposure and strategic function:
· Healthcare personnel (prioritized according to the Armed Forces, security forces, and prison staff)
· Teaching and non-teaching staff (preschool, primary, and secondary)
· Other strategic populations defined by jurisdictions and dose availability
Risk of serious illness:
· Adults aged 70 and over.
· Elderly people living in long-term care facilities.
· Adults aged 60 to 69.
· Adults aged 18 to 59 in high-risk groups.
And taking into account vulnerability criteria: low-income neighborhoods, homeless people, indigenous peoples, prisoners, migrants, university professors, and other groups.