doi: 10.62486/agmu202334
SYSTEMATIC REVIEW
Efficacy of influenza vaccine in adults. Systematic review
Eficacia de la vacuna antigripal en adultos. Revisión sistemática
Yasmin González1
1Universidad Abierta Interamericana, Sede Rosario – Santa Fe, Argentina.
Cite as: González Y. Efficacy of influenza vaccine in adults. Systematic review. Multidisciplinar (Montevideo). 2023; 1:34. https://doi.org/10.62486/agmu202334
Submitted: 04-06-2023 Revised: 10-09-2023 Accepted: 22-11-2023 Published: 23-11-2023
Editor: Telmo
Raúl Aveiro-Róbalo ![]()
ABSTRACT
This study addresses the challenges and advances in the vaccination of older adults in a context of global population aging, evaluating its effectiveness in the prevention of serious infectious diseases. Immunosenescence and comorbidities in this population may affect the response to vaccines, decreasing their efficacy. Through a systematic review, we analyzed recent studies evaluating various vaccine formulations and strategies such as high doses, natural adjuvants (e.g., probiotics and nondigestible polysaccharides), and vaccine coadministration to optimize immunogenicity in the elderly. The results show that high-dose quadrivalent vaccines (QIV-HD) and formulations with natural adjuvants improve immune response and reduce hospitalizations compared to standard doses. Coadministration of vaccines, such as influenza and COVID-19, is a safe practice that does not compromise the efficacy of each vaccine and facilitates vaccination in this high-risk population. The findings support the use of tailored vaccination strategies and underscore the need for additional studies to establish evidence-based recommendations for immunization in older adults.
Keywords: Aged; Adults; Influenza Vaccines; Vaccine Efficacy.
RESUMEN
Este estudio aborda los desafíos y avances en la vacunación de adultos mayores en un contexto de envejecimiento poblacional global, evaluando su efectividad en la prevención de enfermedades infecciosas graves. La inmunosenescencia y las comorbilidades en esta población pueden afectar la respuesta a las vacunas, disminuyendo su eficacia. Mediante una revisión sistemática, se analizaron estudios recientes que evalúan diversas formulaciones de vacunas y estrategias como dosis altas, adyuvantes naturales (por ejemplo, probióticos y polisacáridos no digestibles) y coadministración de vacunas para optimizar la inmunogenicidad en personas mayores. Los resultados muestran que las vacunas cuadrivalentes de alta dosis (QIV-HD) y las formulaciones con adyuvantes naturales mejoran la respuesta inmune y reducen hospitalizaciones en comparación con dosis estándar. La coadministración de vacunas, como la de influenza y COVID-19, es una práctica segura que no compromete la eficacia de cada vacuna y facilita la vacunación en esta población de alto riesgo. Los hallazgos respaldan el uso de estrategias de vacunación adaptadas y subrayan la necesidad de estudios adicionales para establecer recomendaciones basadas en evidencia para la inmunización en adultos mayores.
Palabras clave: Ancianos; Adultos; Vacunas Antigripales; Eficacia de las Vacunas.
INTRODUCTION
Population aging is one of the most important and rapid demographic phenomena of the 21st century. The World Health Organization (WHO) estimates that by 2050, the number of people over 60 will double, reaching nearly 2,1 billion globally.(1,2) This change in population structure poses significant challenges for public health systems, especially in preventing and controlling infectious diseases. One of the most effective and impactful preventive interventions in reducing morbidity and mortality associated with infectious diseases is vaccination. However, the effectiveness of vaccines in the elderly population is not uniform and can be affected by multiple factors related to aging, such as immunosenescence and comorbidities.(3,4)
Immunosenescence, defined as the gradual deterioration of the immune system associated with age, represents an obstacle to the effectiveness of vaccines in older adults. This process involves a decline in innate and adaptive immune responses, reducing the ability of older individuals to generate effective and lasting immune responses after vaccine administration.(5,6) In addition, the coexistence of chronic diseases such as diabetes, cardiovascular disease, and chronic obstructive pulmonary disease (COPD) is common in this population, which can further complicate the response to vaccination and, in some cases, limit the effectiveness of preventive interventions.(6,7,8)
In recent years, research on vaccine efficacy in the elderly population has gained prominence, especially in the context of diseases such as seasonal influenza, pneumococcus, and, more recently, COVID-19.(4) Studies suggest that although vaccines may be less effective in older adults than in younger adults, they continue to offer significant benefits in terms of reducing hospitalizations, serious complications, and mortality.(9,10,11) However, immune response rates and levels of protection vary considerably between different types of vaccines and between older individuals, making it necessary further to analyze vaccine efficacy specific to this age group.(12,13)
The present study aims to systematically review the literature on vaccine efficacy in older adults to provide an updated overview of the immune response and protection conferred in this population.
One of the emerging areas in the field of immunization in older adults is the use of vaccines with adjuvants and high-dose formulations, which have been shown to improve immunogenicity in older people, offering enhanced protection compared to conventional vaccines. Likewise, strategies such as revaccination or periodic booster doses have also been explored in some studies as possible measures to optimize vaccine efficacy in this age group. However, evidence is still limited, and additional studies are needed to establish more straightforward, evidence-based guidelines.(14)
As healthcare systems face pressure from an aging population and an increase in the prevalence of chronic diseases, it is essential to have robust, evidence-based guidelines for vaccine administration in older adults and to design more effective vaccination policies tailored to the needs of this population, promoting a better quality of life and reducing the burden of infectious diseases on healthcare systems.
METHOD
A search was conducted in the PubMed databases to identify studies evaluating the efficacy of vaccines in adults, especially older adults (≥65 years), published between 2020 and 2024. The search terms included combinations of keywords such as “Influenza Vaccines” and “Vaccine Efficacy.” Peer-reviewed clinical trials in English and Spanish that reported results on efficacy, vaccine characteristics, population, and clinical outcomes, with full-text access and conducted in populations over 65 years of age, were included.
This systematic review followed the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines, ensuring transparency and rigor in each process phase.
RESULTS AND DISCUSSION
The workflow and main results are presented. Nineteen clinical studies were obtained for analysis.
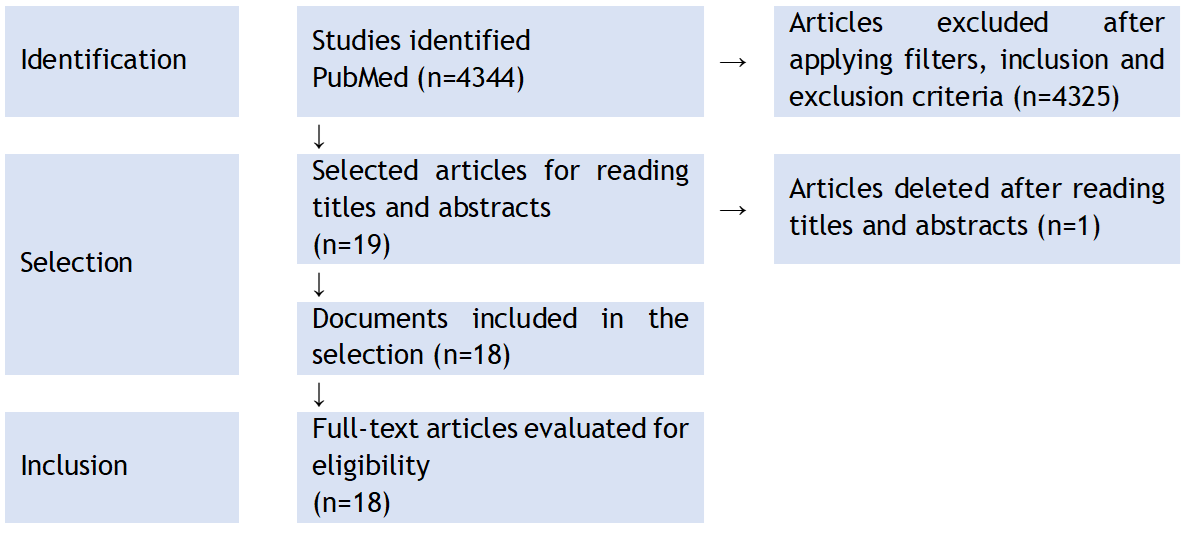
Figure 1. Flowchart of state-of-the-art review according to PRISMA methodology
|
Table 1. Main results of the interventions |
||||
|
Author, year |
Country |
Vaccine |
Population |
Key findings |
|
Curran, 2024(15) |
Multicenter |
RSVPreF3 OA (Respiratory Syncytial Virus Prefusion Protein F) Single dose for older adults, intended to prevent ARI and ETRI |
Adults ≥60 years old 12 466 vaccinated, 12,494 placebo |
71,7 % efficacy against ARI due to RSV; 82,6 % against ETRI due to RSV |
|
Ison, 2024(16) |
Multicenter |
RSVPreF3 OA (RSV Prefusion Protein F) Single dose with optional revaccination; adjuvanted to enhance immunity in older adults |
Adults ≥60 years old 24 967 in combined groups of 2 doses and placebo |
67,2 % efficacy against ETRI by VSR; revaccination did not improve efficacy. |
|
Toback, 2022(17) |
Reino Unido |
NVX-CoV2373 (COVID-19) and seasonal influenza vaccine Co-administration of COVID-19 and influenza vaccines in adults |
Adults (18-64 age subgroup in sub-study) 431 in co-vaccination sub-study |
No significant impact on response to influenza vaccine; COVID-19 efficacy maintained |
|
Shimada, 2021(18) |
Japón |
TIV (Trivalent Influenza Vaccine) + PPSV23 (Pneumococcal Vaccine) Combined influenza and pneumococcal vaccine for patients with CAD |
Adults ≥50 years with coronary artery disease 40 patients with CAD, randomized |
Safe dual vaccine; no significant change in markers after vaccination |
|
Gögenur, 2023(19) |
Dinamarca |
Intratumoral vaccine against Intratumoral, seeks to increase the infiltration of immune cells into tumors |
Patients with colorectal cancer, stages I-III 10 patients with pMMR colorectal tumors |
Increased CD8+ T cell infiltration and PD-L1 expression in tumors |
|
Johansen, 2024(20) |
Dinamarca |
QIV-HD (Quadrivalent High-Dose Influenza) vs QIV-SD (Standard Dose) Comparison of efficacy between high dose and standard dose |
Adults aged 65–79 years 12 477 participants, randomized |
QIV-HD reduced hospitalization rates by 13 % compared to QIV-SD. |
|
Hadigal, 2022(21) |
Europa |
IIV4HD (Quadrivalent High-Dose Influenza) Improves immunogenicity in elderly populations |
Adults ≥60 years old Elderly, without significant comorbidities |
Superior immunogenicity and effectiveness in elderly populations |
|
Leroux-Roels, 2022(22) |
Bélgica |
OVX836 (Nucleoprotein-based influenza vaccine) |
Adults aged 18–65 years 300 healthy adults Nucleoprotein-based, targeting broad-spectrum cellular immunity |
Increased T-cell response; reduction in episodes of influenza-like illness |
|
Alvarez, 2023(23) |
Bélgica, Finlandia, Portugal |
QIV-HD (Quadrivalent High Dose for Influenza) High-dose vaccine for older adults, designed to improve cost-effectiveness and reduce hospitalizations. |
Adults ≥60 years old 3 European countries, ICER and PSA analysis for efficacy and cost |
Cost-effective in older adults; significant reduction in hospitalizations |
|
Shimizu, 2022(24) |
Japón |
Immucise™ Intradermal Vaccine for Influenza Intradermal injection system to maximize immunity with reduced doses in older adults |
Adults ≥65 years old 600 healthy adults, efficacy at 90 and 180 days |
Comparable efficacy to subcutaneous; greater immunity in B strains in ID group |
|
Christensen, 2024(25) |
Dinamarca |
QIV-HD vs QIV-SD Quadrivalent High Dose for Influenza High dose quadrivalent vs standard, relationship between vaccination timing and efficacy |
Adults aged 65–79 12 477 participants, association between ToV and hospitalization |
Early vaccination with QIV-HD reduces hospitalization for pneumonia |
|
Wall, 2021(26) |
Reino Unido |
TIV (Trivalent Influenza) and PPSV23 (Pneumococcal) Vaccines for CKD in people over 65, varied immune responses |
Adults ≥65 years with CRF 65 participants with CRF and healthy controls |
Low immune responses, impact of previous CMV and PPV23 |
|
Uthoff, 2023(27) |
Alemania |
Complex Intervention to Improve Vaccination Rates in General Medicine Training in communication and vaccination processes for general practitioners treating patients over 60 years of age |
Adults ≥60 years old in general medical practices 1057 general medical practices, training interventions |
Increased vaccination rates, improved health literacy |
|
Eto, 2024(28) |
Japón |
Ad26.RSV.preF for VSR Adenovirus-based vaccine for adults ≥60 years of age, evaluating safety and immune response |
Adults ≥60 years old 36 Japanese elderly adults, immunogenicity analysis |
High tolerability and immune efficacy for RSV up to 183 days |
|
Tuthill, 2023(29) |
EE. UU. |
Thymalfasin (Ta1) as an adjunct for COVID-19 Adjuvant for COVID-19 in patients with ESRD, improves immune response |
Hemodialysis patients ≥60 years old 194 hemodialysis patients in Kansas City, compared with a control group |
3 deaths in Ta1 compared to 7 in control; 91 % vaccinated against COVID-19 in group A |
|
Yu, 2020(30) |
EE. UU. |
Experimental adjuvanted vaccine for RSV Adjuvanted vaccine with symptom-based evaluation and virological confirmation |
Adults ≥60 years old 1900 participants, with self-reported symptoms |
Definition of ARI showed good reliability and increased detection with self-sampling. |
|
Sandionigi, 2022(31) |
Italia |
Influenza vaccine + Multi-strain probiotic Seasonal influenza vaccine, administered together with probiotics as a natural adjuvant. |
Adults aged 60 to 80 50 elderly participants vaccinated against influenza |
Significant reduction in infectious symptoms in the probiotic group at 28 and 56 days |
|
Laue, 2021(32) |
Alemania |
Influenza Vaccine + Non-Digestible Polysaccharides Influenza vaccine with polysaccharide adjuvant, in a preparation of β-glucans and arabinoxylans. |
Adults aged 50 to 79 years 239 healthy adults divided into 5 adjuvant groups |
Increase in antibody titers and protection with arabinoxylan; trend toward lower incidence of colds |
|
Note: Legend: ARI Acute respiratory infection, LRTI Lower respiratory tract infection, RSV Respiratory syncytial virus, CAD Coronary artery disease, ICER Incremental cost-effectiveness ratio, PSA Probabilistic sensitivity analysis, CKD Chronic kidney disease, CMV Cytomegalovirus. |
||||
The RSVPreF3 OA and Ad26.RSV.preF vaccines are designed for older adults and show significant effectiveness in reducing acute respiratory infections associated with RSV (ARI-RSV) and lower respiratory tract diseases. In the case of RSVPreF3 OA, 71,7 % effectiveness against ARI-RSV and 82,6 % against ETRI was observed, while Ad26.RSV.preF achieved high tolerability and maintained a long-term immune response up to 183 days post-vaccination.(31) These vaccines are especially relevant for older adult populations due to their efficacy in preventing serious respiratory diseases, which often significantly impact this population. Revaccination, however, did not show additional improvements in some studies, suggesting that immunity induced by a single dose is adequate in most cases.
The studies included several high-dose quadrivalent (QIV-HD) and trivalent vaccine formulations with different adjuvants and approaches to increase immunogenicity in older adults. QIV-HD, used in multiple studies, has been shown to reduce hospitalizations by 13 % compared to standard doses. Adding multistrain probiotics or non-digestible polysaccharides (such as β-glucans and arabinoxylans) significantly improved the immune response and reduced symptoms of common infections, such as colds and flu.(31,32) The increased response regarding immunogenicity and prolonged protection is consistent with growing efficacy in immunosenescent populations. Combinations of QIVs with natural adjuvants, such as probiotics and β-glucans, offer immunomodulatory benefits without the side effects of synthetic adjuvants, making these options attractive for individuals with increased susceptibility to infections and comorbidities.
Co-administration of vaccines, such as NVX-CoV2373 for COVID-19 and influenza vaccines, has been shown not to compromise the efficacy of COVID-19 immunization, indicating that the combination is safe and practical. In addition, no interference with the influenza vaccine response was observed.(31) The ability to administer combination vaccines without diminishing their efficacy is advantageous from a logistical and public health perspective. It facilitates vaccination in high-risk populations during high incidences of respiratory infections. This co-administration represents a practical option for maximizing protection without increasing the number of medical visits.
Intratumoral vaccines (influenza) were tested in patients with colorectal cancer, and other adjuvanted formulations were tested in older adults with special conditions (e.g., chronic renal failure and dialysis treatments). Natural adjuvants, such as Thymalfasin (Ta1) and non-digestible polysaccharides, were safe and positively affected immunity and reduced symptoms of common respiratory infections.(32) In populations with compromised immune conditions, such as dialysis patients, adjuvants may play a key role in improving the response to vaccination without overburdening the immune system. Specific adjuvants such as Ta1 are particularly beneficial, as they facilitate an adaptive immune response without significantly increasing the risk of chronic inflammation.
A systematic comparison of these vaccines highlights that adjuvanted and high-dose formulations, as well as the inclusion of probiotics and non-digestible polysaccharides, have improved vaccine efficacy and immunogenicity in older adults and vulnerable populations. Vaccine co-administration strategies also offer logistical benefits without compromising immunity. The optimal formulation choice should consider the target population's immunological characteristics, with natural adjuvants and high doses recommended for older adults and people with comorbidities.
CONCLUSIONS
High-dose quadrivalent vaccines (QIV-HD) are more effective than standard doses in preventing hospitalizations and severe respiratory illness in older adults, suggesting their preferred use in at-risk populations during seasons of high influenza circulation. In addition, natural adjuvants, such as probiotics and non-digestible polysaccharides, enhance the immune response without significant adverse effects, making it a safe and beneficial option for boosting immunity in older adults with immunosenescence.
Co-administration of vaccines, such as the combination of COVID-19 and influenza vaccines, does not adversely affect immunogenicity and facilitates immune coverage without needing multiple appointments. Likewise, specific adjuvants, such as Thymalfasin (Ta1), show great potential for improving immunity in immunocompromised individuals. Overall, this review supports the use of high doses and natural adjuvants in vaccine formulations and strategic co-administration to maximize immune protection in vulnerable populations, highlighting the importance of further research into personalized combinations to improve effectiveness in high-risk settings.
BIBLIOGRAPHIC REFERENCES
1. Organización Mundioal de la Salud. Envejecimiento. Organ Mundioal Salud 2024. https://www.who.int/es/health-topics/ageing
2. Organización Mundioal de la Salud. WHO: Number of people over 60 years set to double by 2050; major societal changes required. Organ Mundioal Salud 2015. https://www.who.int/news/item/30-09-2015-who-number-of-people-over-60-years-set-to-double-by-2050-major-societal-changes-required.
3. King JC. Influenza vaccines. Pediatr Ann 2000;29:692-7. https://doi.org/10.3928/0090-4481-20001101-09.
4. Youhanna J, Tran V, Hyer R, Domnich A. Immunogenicity of Enhanced Influenza Vaccines Against Mismatched Influenza Strains in Older Adults: A Review of Randomized Controlled Trials. Influenza Other Respir Viruses 2024;18:e13286. https://doi.org/10.1111/irv.13286.
5. Rivetti D, Jefferson T, Thomas R, Rudin M, Rivetti A, Di Pietrantonj C, et al. Vaccines for preventing influenza in the elderly. Cochrane Database Syst Rev 2006:CD004876. https://doi.org/10.1002/14651858.CD004876.pub2.
6. Comber L, O Murchu E, Jordan K, Hawkshaw S, Marshall L, O’Neill M, et al. Systematic review of the efficacy, effectiveness and safety of high-dose seasonal influenza vaccines for the prevention of laboratory-confirmed influenza in individuals ≥18 years of age. Rev Med Virol 2023;33:e2330. https://doi.org/10.1002/rmv.2330.
7. Addario A, Célarier T, Bongue B, Barth N, Gavazzi G, Botelho-Nevers E. Impact of influenza, herpes zoster, and pneumococcal vaccinations on the incidence of cardiovascular events in subjects aged over 65 years: a systematic review. GeroScience 2023;45:3419-47. https://doi.org/10.1007/s11357-023-00807-4.
8. Fiore AE, Bridges CB, Cox NJ. Seasonal influenza vaccines. Curr Top Microbiol Immunol 2009;333:43-82. https://doi.org/10.1007/978-3-540-92165-3_3.
9. Jefferson T, Di Pietrantonj C, Al-Ansary LA, Ferroni E, Thorning S, Thomas RE. Vaccines for preventing influenza in the elderly. Cochrane Database Syst Rev 2010:CD004876. https://doi.org/10.1002/14651858.CD004876.pub3.
10. Osterholm MT, Kelley NS, Sommer A, Belongia EA. Efficacy and effectiveness of influenza vaccines: a systematic review and meta-analysis. Lancet Infect Dis 2012;12:36-44. https://doi.org/10.1016/S1473-3099(11)70295-X.
11. Kim Y-H, Hong K-J, Kim H, Nam J-H. Influenza vaccines: Past, present, and future. Rev Med Virol 2022;32:e2243. https://doi.org/10.1002/rmv.2243.
12. Domnich A, de Waure C. Comparative effectiveness of adjuvanted versus high-dose seasonal influenza vaccines for older adults: a systematic review and meta-analysis. Int J Infect Dis IJID Off Publ Int Soc Infect Dis 2022;122:855-63. https://doi.org/10.1016/j.ijid.2022.07.048.
13. Tanner AR, Dorey RB, Brendish NJ, Clark TW. Influenza vaccination: protecting the most vulnerable. Eur Respir Rev Off J Eur Respir Soc 2021;30:200258. https://doi.org/10.1183/16000617.0258-2020.
14. Lee JKH, Lam GKL, Shin T, Samson SI, Greenberg DP, Chit A. Efficacy and effectiveness of high-dose influenza vaccine in older adults by circulating strain and antigenic match: An updated systematic review and meta-analysis. Vaccine 2021;39 Suppl 1:A24-35. https://doi.org/10.1016/j.vaccine.2020.09.004.
15. Curran D, Matthews S, Cabrera ES, Pérez SN, Breva LP, Rämet M, et al. The respiratory syncytial virus prefusion F protein vaccine attenuates the severity of respiratory syncytial virus-associated disease in breakthrough infections in adults ≥60 years of age. Influenza Other Respir Viruses 2024;18:e13236. https://doi.org/10.1111/irv.13236.
16. Ison MG, Papi A, Athan E, Feldman RG, Langley JM, Lee D-G, et al. Efficacy and Safety of Respiratory Syncytial Virus (RSV) Prefusion F Protein Vaccine (RSVPreF3 OA) in Older Adults Over 2 RSV Seasons. Clin Infect Dis Off Publ Infect Dis Soc Am 2024;78:1732-44. https://doi.org/10.1093/cid/ciae010.
17. Toback S, Galiza E, Cosgrove C, Galloway J, Goodman AL, Swift PA, et al. Safety, immunogenicity, and efficacy of a COVID-19 vaccine (NVX-CoV2373) co-administered with seasonal influenza vaccines: an exploratory substudy of a randomised, observer-blinded, placebo-controlled, phase 3 trial. Lancet Respir Med 2022;10:167-79. https://doi.org/10.1016/S2213-2600(21)00409-4.
18. Shimada K, Morinaga H, Kiyanagi T, Miyazaki T, Nishitani-Yokoyama M, Okai I, et al. Safety and Efficacy of Simultaneous Inoculations of Pneumococcal and Influenza Vaccines in Patients with Coronary Artery Disease. J Atheroscler Thromb 2021;28:826-34. https://doi.org/10.5551/jat.58297.
19. Gögenur M, Balsevicius L, Bulut M, Colak N, Justesen TF, Fiehn A-MK, et al. Neoadjuvant intratumoral influenza vaccine treatment in patients with proficient mismatch repair colorectal cancer leads to increased tumor infiltration of CD8+ T cells and upregulation of PD-L1: a phase 1/2 clinical trial. J Immunother Cancer 2023;11:e006774. https://doi.org/10.1136/jitc-2023-006774.
20. Johansen ND, Modin D, Skaarup KG, Nealon J, Samson S, Dufournet M, et al. Effectiveness of high-dose versus standard-dose quadrivalent influenza vaccine against recurrent hospitalizations and mortality in relation to influenza circulation: A post-hoc analysis of the DANFLU-1 randomized clinical trial. Clin Microbiol Infect Off Publ Eur Soc Clin Microbiol Infect Dis 2024;30:1453-9. https://doi.org/10.1016/j.cmi.2024.01.017.
21. Hadigal S, Colombo L, Haughie S. Reply letter to «Immunogenicity and safety of a quadrivalent high-dose inactivated influenza vaccine compared with a standard-dose quadrivalent influenza vaccine in healthy people aged 60 years or older: a randomized Phase III trial». Hum Vaccines Immunother 2022;18:2085470. https://doi.org/10.1080/21645515.2022.2085470.
22. Leroux-Roels I, Waerlop G, Tourneur J, De Boever F, Maes C, Bruhwyler J, et al. Randomized, Double-Blind, Reference-Controlled, Phase 2a Study Evaluating the Immunogenicity and Safety of OVX836, A Nucleoprotein-Based Influenza Vaccine. Front Immunol 2022;13:852904. https://doi.org/10.3389/fimmu.2022.852904.
23. Alvarez FP, Chevalier P, Borms M, Bricout H, Marques C, Soininen A, et al. Cost-effectiveness of influenza vaccination with a high dose quadrivalent vaccine of the elderly population in Belgium, Finland, and Portugal. J Med Econ 2023;26:710-9. https://doi.org/10.1080/13696998.2023.2194193.
24. Shimizu S, Tanaka R, Itoh E, Maekawa-Matsuura M, Iwase Y. Performance and usability evaluation of novel intradermal injection device ImmuciseTM and reanalysis of intradermal administration trials of influenza vaccine for the elderly. Vaccine 2022;40:873-9. https://doi.org/10.1016/j.vaccine.2021.12.061.
25. Christensen J, Johansen ND, Janstrup KH, Modin D, Skaarup KG, Nealon J, et al. Time of day for vaccination, outcomes, and relative effectiveness of high-dose vs. standard-dose quadrivalent influenza vaccine: A post hoc analysis of the DANFLU-1 randomized clinical trial. J Infect 2024;89:106276. https://doi.org/10.1016/j.jinf.2024.106276.
26. Wall N, Godlee A, Geh D, Jones C, Faustini S, Harvey R, et al. Latent Cytomegalovirus Infection and Previous Capsular Polysaccharide Vaccination Predict Poor Vaccine Responses in Older Adults, Independent of Chronic Kidney Disease. Clin Infect Dis Off Publ Infect Dis Soc Am 2021;73:e880-9. https://doi.org/10.1093/cid/ciab078.
27. Uthoff SAK, Zinkevich A, Franiel D, Below M, Splieth H, Iwen J, et al. A complex intervention on vaccination uptake among older adults (≥ 60 years) in Germany - a study protocol with a mixed methods design. BMC Prim Care 2023;24:148. https://doi.org/10.1186/s12875-023-02101-w.
28. Eto T, Okubo Y, Momose A, Tamura H, Zheng R, Callendret B, et al. A Randomized, Double-Blind, Placebo-Controlled, Phase 1 Study to Evaluate the Safety, Reactogenicity, and Immunogenicity of Single Vaccination of Ad26.RSV.preF-Based Regimen in Japanese Adults Aged 60 Years and Older. Influenza Other Respir Viruses 2024;18:e13336. https://doi.org/10.1111/irv.13336.
29. Tuthill CW, Awad A, Parrigon M, Ershler WB. A pilot trial of Thymalfasin (Ta1) to prevent covid-19 infection and morbidities in renal dialysis patients: Preliminary report. Int Immunopharmacol 2023;117:109950. https://doi.org/10.1016/j.intimp.2023.109950.
30. Yu L, Han Y, Zhang Z. Research on h-index Optimization Considering the Amount of Papers ———hq2-index; [考虑载文量影响的 h 指数优化研究———hq2 指数]. J Mod Inf 2020;40:114-21. https://doi.org/10.3969/j.issn.1008-0821.2020.02.013.
31. Sandionigi A, De Giani A, Tursi F, Michelotti A, Cestone E, Giardina S, et al. Effectiveness of Multistrain Probiotic Formulation on Common Infectious Disease Symptoms and Gut Microbiota Modulation in Flu-Vaccinated Healthy Elderly Subjects. BioMed Res Int 2022;2022:3860896. https://doi.org/10.1155/2022/3860896.
32. Laue C, Stevens Y, van Erp M, Papazova E, Soeth E, Pannenbeckers A, et al. Adjuvant Effect of Orally Applied Preparations Containing Non-Digestible Polysaccharides on Influenza Vaccination in Healthy Seniors: A Double-Blind, Randomised, Controlled Pilot Trial. Nutrients 2021;13:2683. https://doi.org/10.3390/nu13082683.
FINANCING
The authors did not receive funding for the development of this research.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORSHIP CONTRIBUTION
Conceptualization: Yasmin González.
Data curation: Yasmin González.
Formal analysis: Yasmin González.
Research: Yasmin González.
Methodology: Yasmin González.
Project management: Yasmin González.
Resources: Yasmin González.
Software: Yasmin González.
Supervision: Yasmin González.
Validation: Yasmin González.
Visualization: Yasmin González.
Writing – original draft: Yasmin González.
Writing – review and editing: Yasmin González.