doi: 10.62486/agmu202448
CASE REPORT
Cerebral meningitis due to tuberculoma and Epstein Barr: presentation of a clinical case
Meningitis cerebral por tuberculoma y Epstein Barr: presentación de un caso clínico
Elizabeth Gutiérrez Garcia1 ![]() *,
Rommer Alex Ortega Martinez2
*,
Rommer Alex Ortega Martinez2 ![]() *, Masziel Andrea Calle Vilca3
*, Masziel Andrea Calle Vilca3 ![]() *
*
1Medico Residente de medicina crítica y terapia intensiva del Hospital Obrero Nro. 2 de la Caja Nacional de Salud. Cochabamba, Bolivia.
2Medico internista e intensivista. Coordinador de investigación clínica en la Universidad privada del Valle. Hospital Obrero Nro. 2 de la Caja Nacional de Salud. Cochabamba, Bolivia.
3Estudiante de la carrera de medicina de la Universidad Privada del Valle. Cochabamba, Bolivia.
Cite as: Gutiérrez Garcia E, Ortega Martinez RA, Calle Vilca MA. Cerebral meningitis due to tuberculoma and Epstein Barr: presentation of a clinical case. Multidisciplinar (Montevideo). 2024; 2:48. https://doi.org/10.62486/agmu202448
Submitted: 12-10-2023 Revised: 10-02-2024 Accepted: 30-08-2024 Published: 31-08-2024
Editor: Telmo Raúl
Aveiro-Róbalo ![]()
Corresponding Author: Elizabeth Gutiérrez Garcia *
ABSTRACT
Neuroinfection is an inflammatory process that affects the meninges or brain parenchyma; it has various etiologies, including viral and non-viral, including autoimmune, bacterial and fungal; patients with this pathology represent a challenge for doctors; the severity varies, from benign, self-limiting to life-threatening. The Epstein-Barr virus (EBV) is a double-stranded DNA gamma herpesvirus that presents a latent infection and lytic replication; it can diffuse into the central nervous system and alter the integrity of the blood-brain barrier, being associated with neurocognitive impairment, neuronal damage and inflammation. In relation to tuberculosis, it became the second infectious disease that caused the most deaths in the world after COVID-19; tuberculous meningitis is considered the most severe form of extrapulmonary tuberculosis (TB) with a mortality of 70 % in low-income countries. Below is the case of a 35-year-old man with a history of adrenal insufficiency and hyperthyroidism, who was receiving corticosteroids; he went to the emergency service with a 5-month history of clinical symptoms characterized by holocranial headache, focal retrograde amnesia, periods of altered state of consciousness, dizziness, nausea that led to vomiting on several occasions, with sudden loss of consciousness. , accompanied by involuntary tonic-clonic movements and hearing loss; after the diagnostic screening, EBV and TB are identified; receives specific treatment with good clinical evolution.
Keywords: Epstein-Barr Virus Infections; Encephalitis; Meningitis; Mycobacterium Tuberculosis; Spectroscopy; Tuberculoma.
RESUMEN
La neuroinfección es un proceso inflamatorio que afecta a las meninges o del parénquima cerebral; tiene diversas etiologías, incluidas las virales y no virales, entre ellas autoinmunes, bacterianas y fúngicas; los pacientes con esta patología representan un desafío para los médicos; la gravedad varia, desde benigna, autolimitada hasta potencialmente mortal. El virus de Epstein-Barr (VEB) es un herpesvirus gamma de doble cadena de ADN, presenta una infección latente y de replicación lítica; puede difundirse en el sistema nervioso central y alterar la integridad de la barrera hematoencefálica, asociándose con deterioro neurocognitivo, daño neuronal e inflamación. En relación con la tuberculosis, se convirtió en la segunda enfermedad infecciosa que más muertes causó en el mundo después de la COVID-19; la meningitis tuberculosa se considera la forma más grave de tuberculosis (TBC) extrapulmonar con una mortalidad del 70 % en los países de ingresos bajos. A continuación se presenta el caso de un varón de 35 años con antecedentes de insuficiencia suprarrenal e hipertiroidismo, que recibía corticoides; acude al servicio de emergencia con cuadro clínico de 5 meses de evolución caracterizado por presentar cefalea holocraneana, amnesia retrograda focal, periodos de alteración del estado de la consciencia, mareos, nauseas que llegan al vomito en varias oportunidades, con perdida súbita del estado de conciencia, acompañado de movimientos involuntarios de tipo tonico-clonicos e hipoacusia; tras la pesquisa diagnostica se identifica VEB y TBC; recibe tratamiento específico con buena evolución clínica.
Palabras clave: Meningitis; Micobacterium Tuberculosis; Infecciones por Virus de Epstein-Barr; Tuberculoma; Encefalitis; Espectroscopia.
INTRODUCTION
Infectious processes affecting the nervous system are often a medical emergency, and recognizing them early is a challenge; thus, having a high level of suspicion of a neuroinfection for early identification and initiation of appropriate treatment will reduce the risk of morbidity and mortality. Meningitis and encephalitis can present with undifferentiated encephalopathy and can cause a variety of complications due to secondary injury to the structures of the nervous system(1) where encephalitis is an inflammation of the brain parenchyma with viral and non-viral etiologies, including autoimmune, bacterial and fungal, whose timely management is essential, due to the high risk of mortality and severe neurological sequelae; on the other hand, meningitis is an infection of the central nervous system, which causes inflammation of the meninges, the protective layer that covers the brain and the spinal column; bacterial, viral, fungal and non-infectious etiologies cause it. Despite their differences in physiopathology and epidemiology, the clinical presentations could be so similar that they result in a possible erroneous diagnosis.(2,3)
Tuberculosis is one of the oldest human pathogens(4) caused by Mycobacterium tuberculosis, a bacterium that almost always affects the lungs and is transmitted from person to person through the air;(5) dissemination from the lungs is a rare event that leads to extrapulmonary tuberculosis.(4) In 2022, tuberculosis became the second most deadly infectious disease worldwide after COVID-19 and one of the leading causes of death among people with human immunodeficiency virus; globally, an estimated 10,6 million people fell ill with tuberculosis, and 1,3 million died from it.(5)
In Latin America, for 2022, an estimated 325 000 new tuberculosis cases were reported, and 239 987 (74 %) were reported. It is predicted that around 25 % of all tuberculosis cases are extrapulmonary;(6) according to Behr et al., it is attributed to 15 %.(7) In Bolivia, the estimated incidence of tuberculosis per 100 000 inhabitants was 107,5 cases, with 1372 deaths by 2022. However, there is no data on extrapulmonary tuberculosis, especially at the neurological level.(5) Although cases of extrapulmonary tuberculosis affect different organs (lymph nodes, pleura, bones and joints, abdomen, meninges, and genitourinary tract), tuberculous meningitis is considered the most serious form, with a mortality rate of up to 70 % in low-income countries.(6)
Epstein-Barr virus is a double-stranded DNA-containing gamma herpesvirus(8) belonging to the Herpesviridae family. It is the causative agent of infectious mononucleosis and has also been linked to various tumors such as nasopharyngeal and gastric carcinomas, Burkitt's lymphoma, Hodgkin's disease, T-cell lymphoma and B-cell lymphoma and smooth muscle tumors;(9) more than 90 % of the world's adult population is chronically infected with this virus(10) with infection being common in early childhood and a second peak in frequency in late adolescence; in adulthood, more than 90 % of individuals become infected with the virus; on the other hand, it is spread through contact with oral secretions and its dissemination increases in immunodeficient patients and those with infectious mononucleosis.(9) The nervous system is clinically involved in Epstein-Barr virus infection in 0,5 ± 7,5 % of cases; of these, more than 25 % have abnormalities of the cerebrospinal fluid; neurological manifestations range from meningitis, encephalitis, cranial nerve palsies, myelitis, anterior horn syndrome, polyradiculitis, polyneuropathy, mononeuropathy.(11)
The clinical findings in a neuroinfection include fever, headache, photophobia, phonophobia, stiff neck, nausea, vomiting, arthralgia, myalgia, rash, abdominal pain, irritability, sore throat, and altered mental state.(12) Likewise, they should undergo a lumbar puncture (LP) and evaluation of the cerebrospinal fluid (CSF) for a definitive diagnosis, associated with imaging studies such as a cranial tomography with and without contrast and magnetic resonance, in this case, if available by spectroscopy.(3)
Regarding treatment, it is essential to implement it in a specific and early manner to avoid developing secondary complications such as neurological sequelae. In this way, it is advisable to analyze the relationship between neuro infection, secondary to viral diseases, and mycobacterium tuberculosis, even more so if the patients are immunocompromised, as presented below.
Presentation of the clinical case
The patient is evaluated as being alert, conscious, oriented in time, space, and person, with dysarthria and right brachial paresis, full moon face; he reports holo cranial headache, intensity 10/10 on the visual analog scale for pain (VAS); otherwise, without particularities. He had an electroencephalogram (EEG) performed privately 6 days ago with no epileptiform activity or signs of focal involvement. He is admitted to the general ward with follow-up by endocrinology and neurology; a patient is described as having a pulsatile, moderate intensity, unilateral headache, as well as mild right motor impairment, associated with nausea and vomiting that is exacerbated by exertion, possibly compatible with a migraine headache and a previous ischemic cerebrovascular accident (CVA); he was medicated with amitriptyline and valproic acid. Simple cranial tomography (CT) scan with ischemic sequelae in the anterior arm of the left internal capsule; despite the treatment started, the patient presented further neurological deterioration. However, the family requested voluntary discharge.
Twenty-four hours after being discharged, he is brought back to the emergency department on a stretcher in a state of superficial stupor with hypertonia in the upper extremities and neck stiffness, but with negative Kernig and Brudzinski signs (Glasgow 10/15), carrying a report from a private clinic that highlights the start of antibiotic therapy (ceftriaxone 2 grams every 12 hours) after a lumbar puncture obtaining cerebrospinal fluid (CSF) of clean color before centrifugation, clean after centrifugation, density 1020, pH: neutral (7,0) coagulability 0 %, Ziehl Neelsen staining was performed negative (-).
He is admitted to the general ward with suspected meningeal syndrome, hypothyroidism, and previous ischemic stroke. In the next few hours, he presents with more excellent neurological compromise, which is why they request an assessment by the intensive care unit (ICU). A patient is found in a deep stupor (Glasgow 7/15 RV:1, RO:2 RM 4), with isochoric reactive pupils with an upward deviation of the gaze, stiff neck, reflexes of the trunk present, poor management of secretions and respiratory failure with use of accessory muscles; immediately admitted to the ICU, airway protected by orotracheal intubation and initiation of analgesia-sedation and hemodynamic stabilization. A non-contrast CT scan is performed, with known ischemic sequelae and no evidence of intracranial hypertension; subsequently, a sample of cerebrospinal fluid is taken and a cytochemical, cytological, culture, and viral, mycological, bacteriological, and GeneXpert panel for tuberculosis are requested, all of which are reported negative (Table 1); in addition to serological tests for hepatitis B and C; ELISA for HIV (human immunodeficiency virus), also negative. Due to the suspicion of a neuroinfection such as aseptic meningitis, empirical treatment was started with acyclovir 800 mg every 8 hours, ceftriaxone 2 g every 12 hours, vancomycin 1 g every 12 hours and dexamethasone 4 mg every 8 hours. Subsequently, the result of the cytochemical test is obtained, which is compatible with a neuroinfection (table 2).
Patient with favorable evolution, without neurological deterioration (Glasgow 14/15), extubated after 48 hours, after a test of spontaneous ventilation, and given all the data collected from the virological, bacteriological, mycological, and GeneXpert panels, which were negative, and data compatible with neuroinfection in the cerebrospinal fluid (CSF) cytochemical test, treatment was continued. After 72 hours, he again presents neurological deterioration (Glasgow 10/15). A simple cranial tomography is requested (figure 1a) where cerebral edema is evident but with free peritruncal cisterns; the condition is exacerbated with a tonic-clonic convulsive episode, and respiratory failure, use of accessory muscles and poor management of secretions, tachypneic, tachycardic, sub febrile with temperature of 37,7 °C Glasgow 10/15 RO:3 RM: 5 RV: 2, airway protected again under rapid sequence intubation with subsequent connection to mechanical ventilator, initiation of analgosedation for RASS-4. Cultures are taken, and a new lumbar puncture (2nd sample) and cerebrospinal fluid sample for cytochemical analysis are taken, even with infection data (table 2); in addition to cytological, culture, viral panel, mycological, bacteriological, and GeneXpert. Likewise, serology tests are repeated: Hepatitis B, negative for hepatitis C, negative cytomegalovirus IgG and IgM, negative herpes IgG and IgM, negative toxoplasmosis IgG and IgM, and non-reactive HIV viral load.
In the next few hours, Mycobacterium tuberculosis and Epstein Barr are reported in the cerebrospinal fluid (table 1); anti-tuberculosis treatment is immediately started with rifampicin 5 mg/kg per day, isoniazid 10 mg/kg per day, ethambutol 15 mg/kg per day, pyrazinamide 20 mg/kg per day. It is continued with acyclovir 10 mg/kg every 8 hours.
|
Table 1. Real-time polymerase chain reaction (PCR) results for bacteriological, viral, and fungal testing of cerebrospinal fluid (CSF) |
|||
|
Examination |
Identification |
1st sample (17.06.24) |
2nd sample (23.06.24) |
|
Bacterial panel |
Haemophilus influenzae, Streptococcus pneumoniae, Streptococcus group B, Listeria monocytogenes, Neisseria meningitidis, E. coli K1. |
NOT DETECTED |
NOT DETECTED |
|
Mycobacterium tuberculosis and resistance |
Resistance to rifampicin Resistance to isoniazid |
NOT DETECTED |
Positive for MT |
|
Meningitis virus panel |
(Herpes simplex type 1, herpes virus type 2, varicella zoster virus, human herpes virus type 6, human herpes virus type 7, cytomegalovirus, Epstein Barr virus) |
NOT DETECTED |
Positive for Epstein Barr |
|
DNA of Cryptococcus neoformans |
|
NOT DETECTED |
NOT DETECTED |
|
Toxoplasma gondii DNA: not detected |
|
|
NOT DETECTED |
|
DNA: deoxyribonucleic acid. |
|||
|
Table 2. Cytochemical results of cerebrospinal fluid (CSF) Obrero Hospital No. 2 |
||
|
Cerebrospinal fluid |
1st Puncture (06/24/17) |
2nd puncture (06/24/23) |
|
Volume |
1 ml |
5 ml |
|
Appearance: |
Clear |
Slightly opalescent |
|
Color: |
Transparent |
Light yellow |
|
Color after centrifugation |
Transparent |
Light yellow |
|
Hematoxylin stain |
Absent |
Small red |
|
Coagulability |
Absent |
Absent |
|
Glucose: |
20 mg/dl |
29 mg/dl |
|
Proteins |
182 mg/dl |
243 mg/dl |
|
Albumin |
No reagent |
No reagent |
|
LDH: |
73 U/L |
212 U/L |
|
PH |
8 |
7,5 |
|
Density |
1020 |
- |
|
Leukocytes |
10 mm3 |
68 mm3 |
|
Lymphocytes |
100 % |
95 % |
|
PMN: |
- |
5 % |
|
MN |
- |
|
|
LDH: lactate dehydrogenase, PMN: polymorphonuclear, MN: mononuclear, absent blood button: absence of blood cells. |
||
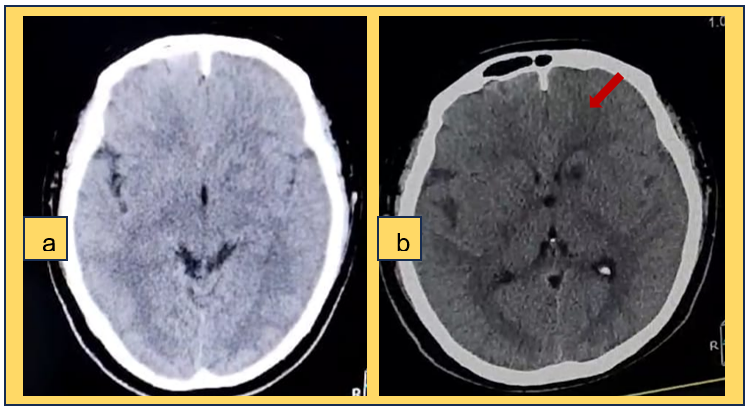
Figure 1. a) Simple skull CT scan b) skull CT scan without contrast showing a hypodense area suggestive of ischemia in the left internal capsule region
Due to neurological deterioration and no imaging evidence of any acute event predisposing the neurological status, and due to the report of neuroinfection by cerebrospinal fluid cytochemistry, a new non-contrast cranial CT scan was performed (figure 1b), showing a hypodense area suggestive of ischemia in the left internal capsule region and decreased cerebral edema. On the other hand, a contrast-enhanced magnetic resonance imaging (MRI) scan was performed, which reported multiple lesions with ring enhancement, diffuse in the cerebral parenchyma, not restricted in diffusion, i.e., with increased cellularity at the level of the lesions, which did not suggest a cerebral abscess (figure 2a-g).
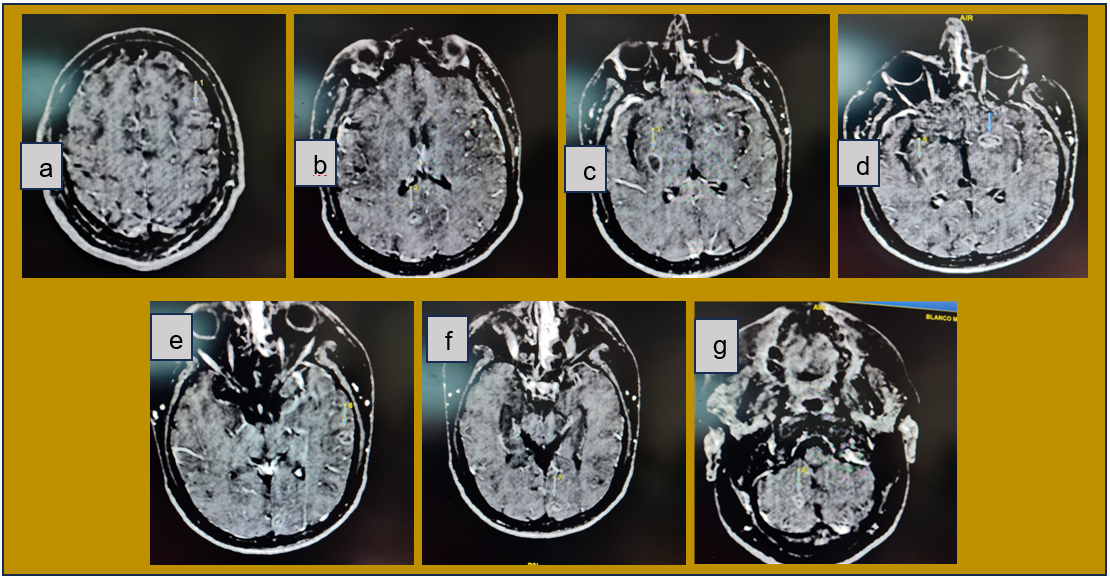
Figure 2. a, b, c, d, e, f, g: multiple supra-infratentorial lesions with ring enhancement and perilesional edema suggestive of tuberculoma, g; similar lesion measuring 12x9 mm in the right cerebellar lobe
Subsequently, an MRI with spectroscopy was performed, which reported elevated N-acetylcholine levels, not compatible with abscess or metastasis (figure 3), and an electroencephalogram reported theta rhythm, symmetry, and no epileptiform events.
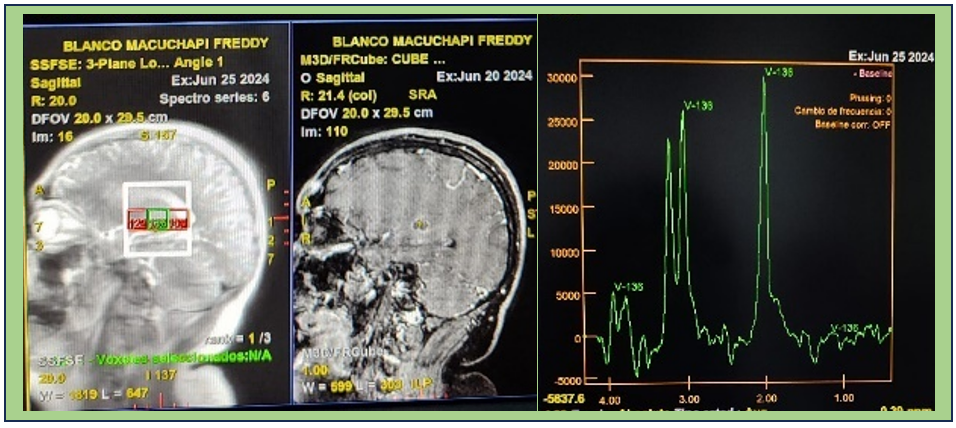
Figure 3. Nuclear magnetic resonance imaging with peak elevation spectroscopy N. acetylcholine, not compatible with bacterial abscess or metastasis
The patient's evolution was characterized by remaining hemodynamically stable, with improved neurological status (Glasgow 14/15), good tolerance to spontaneous ventilation testing, and successful extubation without complications. He underwent rehabilitation physiotherapy and continued specific treatment so that he could be discharged for management and follow-up by infectious disease, neurology, and endocrinology specialists.
DISCUSSION
Central nervous system infection is a medical emergency, but it is challenging for physicians to recognize it early. Therefore, it is essential to maintain an optimal level of suspicion to facilitate the initiation of treatment. This can reduce the risk of morbidity and mortality, as well as complications due to secondary injury, especially in those with impaired immune function.(3,4,5) The patient presented that he has a history of regular prednisone use for more than 5 months secondary to adrenal insufficiency, which is a predisposing factor.
It is essential to differentiate encephalitis from meningitis, although they may overlap. The former is an inflammation of the brain parenchyma(2,7) which may be caused by infection, especially in the gray matter. It is also caused by immune-mediated damage affecting the white matter. In the infectious group, both neurotropic and non-neurotropic pathogens can cause encephalitis(9,10,11,12,13) meningitis causes inflammation of the protective layer covering the brain and spinal cord, caused by bacterial, viral, fungal, and non-infectious etiologies.(2) Despite their differences in pathophysiology and epidemiology, clinical presentations can be so similar that they result in a possible misdiagnosis; however, in the case presented, it is considered meningitis caused by a viral agent and mycobacterium.
In relation to the clinical case, the patient presented with nervous system infection symptoms. A lumbar puncture was performed, and empirical treatment was initiated. A few hours later, the cerebrospinal fluid cytochemical report confirmed the diagnosis. It was classified as aseptic meningitis since there was no bacteriological, mycotic, or viral recovery with negative GeneXpert results. According to Walgreen et al., aseptic meningitis is defined as an acute community-acquired syndrome with cerebrospinal fluid pleocytosis in the absence of positive Gram staining and culture, with no focus on meningitis or systemic disease and with good clinical outcome.(14,15) From another perspective, they can be classified as infectious and non-infectious,(12) with non-infectious cases being the most common; another review classifies them into three main groups: a) systemic diseases with meningeal involvement, b) drug-induced aseptic meningitis, and c) neoplastic meningitis.(15)
Aseptic meningitis is characterized by low and predominantly lymphocytic pleocytosis, normal glucose levels, and normal to slightly elevated protein levels, unlike bacterial meningitis, which has very high and predominantly neutrophilic pleocytosis, low glucose levels, and high protein levels. In this case, the results of the first puncture are highly suggestive of viral meningitis vs. TB rather than bacterial meningitis; however, this may vary in patients with partially treated bacterial meningitis, immunosuppression, or meningitis caused by Listeria monocytogenes.(3)
Most reviews in the literature include groups of meningitis that are not aseptic, being strictly bacterial meningitis; however, they do not present sterile CSF; this may be due to prior administration of antibiotics, as in our case, where the patient received ceftriaxone. From another perspective, it is recommended to evaluate associated para-meningeal infections.(15) It was observed that the patient finally tested positive for two pathogens (Mycobacterium tuberculosis and Epstein-Barr virus) and also showed neurological and clinical improvement at 48 hours, so it is impossible to confirm aseptic meningitis.
Meningitis caused by Mycobacterium tuberculosis is a rare manifestation, accounting for approximately 1 % of all tuberculosis cases.(16) It is considered one of the most devastating types of extrapulmonary tuberculosis because it is associated with high mortality and a high rate of neurological sequelae. Despite this outlook in the clinical case in question, although the patient presented initial neurological deterioration, he showed significant improvement after identifying the mycobacterium and early specific treatment.(17)
In terms of symptoms, CNS tuberculosis presents with nonspecific symptoms such as headache, fever, neck stiffness, vomiting, and occasionally cognitive changes, which is why, according to Schaller et al.(18,19), it can be challenging to diagnose.(4) On the other hand, there is a prodromal period of 2 to 4 weeks with nonspecific symptoms such as fatigue, malaise, myalgia, and fever that precedes CNS tuberculosis; if a meningitic state ensues, the picture is accompanied by severe headache, fever, vomiting, photophobia, and neck stiffness (75 % of cases).(19)
Due to its low frequency, tuberculous meningitis remains a challenge for medical professionals; the disease begins with the development of small tuberculous foci, called Rich foci, in the brain, spinal cord, or meninges; the location of these foci and the ability to control them ultimately determine which form of central CNS tuberculosis presents; these can be tuberculous meningitis, in most cases, followed by tuberculous encephalitis, intracranial tuberculoma, which is suggestive in the case presented; on the other hand, it can present as tuberculous brain abscess, which occurs in fewer cases,(16) and rarely, cerebritis or encephalopathy, which have been described in children.(18)
Patients with tuberculoma also present with headache, vomiting, drowsiness, papilledema, hemiparesis, or seizures, mimicking other space-occupying lesions in the central nervous system, which may explain the patient's significant initial neurological deterioration.(20) The patient has multiple lesions throughout the brain parenchyma, in addition to a history of ischemia in the left internal capsule, which could be differentiated from a tuberculoma due to the symptoms reported for the past 6 months and the current hospitalization, which is consistent with the literature review.
Although meningeal tuberculosis is considered one of the most devastating types of extrapulmonary tuberculosis, with a high rate of neurological sequelae, the complications it can present include cranial nerve palsy in 25 to 50 % of cases (mainly affecting the abducens and oculomotor nerves), altered cerebrospinal fluid circulation, leading to hydrocephalus, altered consciousness, and seizures (10,15 %); parenchymal damage results from infarction due to vasculitis or direct inflammatory involvement of the meninges and brain parenchyma; hemiparesis and altered consciousness are the most common deficits after an infarction related to meningeal tuberculosis, accompanied or not by aphasia or hemianopsia, which were not present in the clinical case; attention should also be paid to the frequent occurrence of hyponatremia; due to salt-losing syndrome or inadequate antidiuretic hormone secretion in 50 % of cases.(19)
In meningeal tuberculosis, CSF shows lymphocytic pleocytosis with an average cell count of around 200 cells/μl (10-1000×103 cells/ml), moderate to severe protein elevation (0,5-3,0 g/l), and glucose levels below 45 mg/dl or below 40 or 50 % of serum glucose (hypoglycorrhachia), as in our clinical case; on the other hand, lactate levels in the cerebrospinal fluid may increase from 5,0 to 10,0 mmol/l.(19) HIV coinfection substantially increases the risk of developing CNS TB, especially in patients with severe immunosuppression (CD4 count <100 cells/μL);(18) in our case, the patient is HIV (-), with a history of corticosteroid use secondary to adrenal insufficiency.
Viral encephalitis is caused by direct viral invasion of the brain parenchyma and presents intracranial hypertension, unlike viral meningitis, which is a diffuse inflammatory syndrome of the pia mater and arachnoid membrane; patients present fever, headache, vomiting, and signs of meningeal irritation, as in the patient mentioned above.(8) The Epstein-Barr virus, also known as human herpesvirus 4,(10) is a double-stranded DNA virus found in 90 to 95 % of the world's population, with a latent infection and lytic replication;(8) 25 % have cerebrospinal fluid abnormalities;(11) It can replicate in the CNS and alter the integrity of the blood-brain barrier (BBB), which is associated with neurocognitive impairment, neuronal damage, and inflammation.(8) Identifying the degree of the patient's involvement due to this virus is complicated because the patient presented with Mycobacterium tuberculosis, and his favorable evolution was secondary to specific treatment for both pathogens.
According to Peter Portegies et al.(11), complications from the Epstein Barr virus range from meningitis, encephalitis, cranial nerve palsy, myelitis, anterior horn syndrome, polyradiculitis, polyneuropathy, mononeuropathy(11), another angle, it contributes to the development of Alzheimer's disease, Parkinson's disease, multiple sclerosis, acute cerebellar ataxia, meningitis, acute disseminated encephalomyelitis, and brain tumors.(8) According to Angelini et al., diagnosis is based on tests for heterologous IgM antibodies against the Epstein Barr virus, IgM seropositivity for viral capsid antigens, and/or PCR in cerebrospinal fluid, associated with suggestive imaging studies.(8) Low socioeconomic status and geographical areas with poor hygiene lead to more infections in children. However, in regions with higher hygiene standards, infection is more common in adults, and infectious mononucleosis is more prevalent.(9)
Modern diagnostic techniques currently include polymerase chain reaction (PCR) testing, which offers high sensitivity and detection of drug resistance;(20) in these cases, determination of intrathecal IgG synthesis can provide supporting evidence to differentiate the diagnosis of tuberculous meningitis from aseptic meningitis (sensitivity 100 %, specificity 83,3 %).(19)
Contrast-enhanced magnetic resonance imaging is considered the modality of choice in the evaluation and detection of central nervous system tuberculosis and is superior to CT;(19) typical features of cerebral tuberculosis present with meningeal enhancement in the basal or supratentorial cistern (tuberculous meningitis); enhanced masses at the edge or nodular (tuberculomas); and atypical findings such as hydrocephalus and abnormal FLAIR signal changes in the infratentorial or cranial nerves; with regard to tuberculomas, they may be noncaseating, which generally show high signal intensity on T2WI and slightly low signal intensity on T1WI; and caseating tuberculomas, which show iso- to high signal intensity, both on T1WI and T2WI, with a peripheral edge of iso- to high signal intensity on T2WI; more in relation to the patient presented;(17) on the other hand, in our case, an MRI with spectroscopy was performed, which ruled out a bacterial abscess or metastasis; this assesses the biochemical characteristics of the tissues and provides metabolic information.(21)
Early detection of CNS tuberculoma warrants an initial treatment strategy; first-line treatment consists of isoniazid, rifampicin, pyrazinamide, and streptomycin or ethambutol; treatment duration varies from 9 to 12 months;(13) after 2 months of therapy with the four drugs, for meningitis caused by susceptible strains, pyrazinamide (PZA) and streptomycin (EMB) can be discontinued. Treatment with isoniazid (INH) and rifampicin (RIF) can be continued for 7 to 10 months. However, the optimal duration of chemotherapy, which the patient received until his follow-up in outpatient care, has not been defined.(22) Regarding treatment for Epstein Barr, the antiviral agents tested against inflammatory meningitis were mainly acyclovir and its prodrug valacyclovir, as received by the patient.(23)
Suspicion of a neuroinfection is essential in cases where the clinical and epidemiological picture is unclear, especially with data on previous antibiotic therapy. Extrapulmonary tuberculosis accounts for a relatively small percentage of human tuberculosis cases in immunocompetent adults; however, it is associated with high morbidity and mortality in immunocompromised patients. where clinical outcomes are truly devastating. That is why timely diagnosis of infections can dramatically improve the likelihood that the disease will respond to treatment. Thanks to timely and specific treatment, there were no serious neurological sequelae in the case presented. On the other hand, identifying a possible tuberculoma is still a challenge in the current literature.
BIBLIOGRAPHIC REFERENCES
1. Jones LK Jr. Neurocritical Care and the Death of Therapeutic Nihilism. Continuum (Minneap Minn). 2024 Jun 1;30(3):554-555. doi: 10.1212/CON.0000000000001460. Continuum (minneap minn) 2024;30(3, neurocritical care): 757–780.
2. Rohani H, Arjmand R, Mozhgani S, Shafiee A, Javad Amini M, Forghani-Ramandi M. The worldwide prevalence of herpes simplex virus encephalitis and meningitis: A systematic review and meta-analysis. Turk Arch Pediatr. 2023;58(6):580-587. http://dx.doi.org/10.5152/turkarchpediatr.2023.23007
3. Mount HR, Boyle SD. Aseptic and Bacterial Meningitis: Evaluation, Treatment, and Prevention. Am Fam Physician. 2017 Sep 1;96(5):314-322. https://pubmed.ncbi.nlm.nih.gov/28925647/
4. Moule MG, Cirillo JD. La diseminación de Mycobacterium tuberculosis juega un papel crítico en la patogénesis. Front Cell Infect Microbiol [Internet]. 2020 ;10. Disponible en: http://dx.doi.org/10.3389/fcimb.2020.00065
5. Situación de la Tuberculosis en las Américas [Internet]. Paho.org. [citado el 20 de agosto de 2024]. Disponible en: https://www.paho.org/es/temas/tuberculosis/situacion-tuberculosis-americas
6. Zürcher K, Ballif M, Kiertiburanakul S, Chenal H, Yotebieng M, Grinsztejn B, et al. Diagnóstico y resultados clínicos de la tuberculosis extrapulmonar en programas de terapia antirretroviral en países de ingresos bajos y medios: un estudio multicohorte. J Int AIDS Soc [Internet]. 2019;22(9). Disponible en: http://dx.doi.org/10.1002/jia2.25392
7. Behr MA, Edelstein PH, Ramakrishnan L. Revisiting the timetable of tuberculosis. BMJ [Internet]. 2018; k2738. Disponible en: http://dx.doi.org/10.1136/bmj.k2738
8. Zhang N, Zuo Y, Jiang L, Peng Y, Huang X, Zuo L. Epstein-Barr virus and neurological diseases. Front Mol Biosci [Internet]. 2022;8. Disponible en: http://dx.doi.org/10.3389/fmolb.2021.816098
9. Cohen, Jeffrey I. «Infecciones causadas por el virus de Epstein-Barr, incluida mononucleosis infecciosa.» Harrison. Principios de Medicina Interna, 20e Eds. J. Larry Jameson, et al. McGraw-Hill Education,2018, https://accessmedicina.mhmedical.com/content.aspx?bookid=2461§ionid=209900925.
10. Wong Y, Meehan MT, Burrows SR, Doolan DL, Miles JJ. Estimating the global burden of Epstein–Barr virus-related cancers. J Cancer Res Clin Oncol [Internet]. 2022 ;148(1):31–46. Disponible en: http://dx.doi.org/10.1007/s00432-021-03824-y
11. Portegies P, Corssmit N. Epstein-Barr virus and the nervous system. Curr Opin Neurol. 2000;13(3):301-304. doi:10.1097/00019052-200006000-00012
12. Leuci S, Coppola N, Cantile T, Calabria E, Mihai LL, Mignogna MD. Aseptic meningitis in oral medicine: Exploring the key elements for a challenging diagnosis: A review of the literature and two case reports. Int J Environ Res Public Health [Internet]. 2022;19(7):3919. Disponible en: http://dx.doi.org/10.3390/ijerph19073919
13. Kumar R. Understanding and managing acute encephalitis. F1000Res [Internet]. 2020;9:60. Disponible en: http://dx.doi.org/10.12688/f1000research.20634.1
14. Shukla B, Aguilera EA, Salazar L, Wootton SH, Kaewpoowat Q, Hasbun R. Aseptic meningitis in adults and children: Diagnostic and management challenges. J Clin Virol. 2017 Sep;94:110-114. doi: 10.1016/j.jcv.2017.07.016. Epub 2017 Aug 4.
15. Tattevin P, Tchamgoué S, Belem A, Bénézit F, Pronier C, Revest M. Aseptic meningitis. Rev Neurol (Paris) [Internet]. 2019;175(7–8):475–80. Disponible en: http://dx.doi.org/10.1016/j.neurol.2019.07.005
16. Rock RB, Olin M, Baker CA, Molitor TW, Peterson PK. Tuberculosis del sistema nervioso central: patogénesis y aspectos clínicos. Clin Microbiol Rev [Internet]. 2008;21(2):243–61. Disponible en: http://dx.doi.org/10.1128/cmr.00042-07
17. Hwang JH, Lee KM, Park JE, Kim H-G, Kim EJ, Choi WS, et al. Atypical Cerebral Manifestations of Disseminated Mycobacterium tuberculosis. Front Neurol [Internet]. 2017;8. Disponible en: http://dx.doi.org/10.3389/fneur.2017.00462
18. Bovijn, L., Solomons, R., Marais, S. (2019). Tuberculosis neurológica en el VIH. En: Sereti, I., Bisson, GP, Meintjes, G. (eds) VIH y tuberculosis. Springer, Cham. https://doi.org/10.1007/978-3-030-29108-2_13
19. Schaller, MA, Wicke, F., Foerch, C. et al. Tuberculosis del sistema nervioso central. Clin Neuroradiol 29 , 3–18 (2019). https://doi.org/10.1007/s00062-018-0726-9
20. De Lance AR, Safaee M, Oh MC, Clark AJ, Kaur G, Sun MZ, et al. Tuberculoma of the central nervous system. J Clin Neurosci [Internet]. 2013;20(10):1333–41. Disponible en: http://dx.doi.org/10.1016/j.jocn.2013.01.008
21. Majós C. Espectroscopia por resonancia magnética de protón en el diagnóstico de tumores cerebrales. Radiologia [Internet]. 2005;47(1):1–12. Disponible en: http://dx.doi.org/10.1016/s0033-8338(05)72790-9
22. Nahid P, Dorman SE, Alipanah N, Barry PM, Brozek JL, Cattamanchi A, et al. Official American thoracic society/Centers for Disease Control and Prevention/Infectious Diseases Society of America clinical practice guidelines: Treatment of drug-susceptible tuberculosis. Clin Infect Dis [Internet]. 2016;63(7):e147–95. Disponible en: http://dx.doi.org/10.1093/cid/ciw376
23. Andersen O, Ernberg I, Hedström AK. Treatment options for Epstein-Barr virus-related disorders of the central nervous system. Infect Drug Resist [Internet]. 2023;16:4599–620. Disponible en: http://dx.doi.org/10.2147/idr.s375624
FINANCING
None.
CONFLICT OF INTEREST
Authors declare that there is no conflict of interest.
AUTHORSHIP CONTRIBUTION
Conceptualization: Elizabeth Gutiérrez Garcia, Rommer Alex Ortega Martinez, Masziel Andrea Calle Vilca.
Data curation: Elizabeth Gutiérrez Garcia, Rommer Alex Ortega Martinez, Masziel Andrea Calle Vilca.
Formal analysis: Elizabeth Gutiérrez Garcia, Rommer Alex Ortega Martinez, Masziel Andrea Calle Vilca.
Research: Elizabeth Gutiérrez Garcia, Rommer Alex Ortega Martinez, Masziel Andrea Calle Vilca.
Methodology: Elizabeth Gutiérrez Garcia, Rommer Alex Ortega Martinez, Masziel Andrea Calle Vilca.
Project management: Elizabeth Gutiérrez Garcia, Rommer Alex Ortega Martinez, Masziel Andrea Calle Vilca.
Supervision: Elizabeth Gutiérrez Garcia, Rommer Alex Ortega Martinez, Masziel Andrea Calle Vilca.
Validation: Elizabeth Gutiérrez Garcia, Rommer Alex Ortega Martinez, Masziel Andrea Calle Vilca.
Visualization: Elizabeth Gutiérrez Garcia, Rommer Alex Ortega Martinez, Masziel Andrea Calle Vilca.
Writing - original draft: Elizabeth Gutiérrez Garcia, Rommer Alex Ortega Martinez, Masziel Andrea Calle Vilca.
Writing - proofreading and editing: Elizabeth Gutiérrez Garcia, Rommer Alex Ortega Martinez, Masziel Andrea Calle Vilca.